The potential of stem cell therapy to revolutionizing medical treatments is immensely promising. However, the path from initial product idea to clinical application can be challenging and time-consuming, largely due to the strict regulatory reviews and requirements involved. That is precisely why AcceGen is here to provides stem cell solutions.
AcceGen’s team of professional scientists is dedicated to expediting the development of stem cell therapy. We offer a comprehensive range of high-quality reagents and materials for every stage of stem cell solution process. Through our services, we collaborate closely with you to develop the best solution that aligns with your specific requirements and prioritize keeping you informed and involved, ensuring transparency and effective communication regarding the progress and milestones achieved of the project.
-
Stem cell isolation and expansion are crucial steps in stem cell research and therapy. These processes involve source selection, isolation, culture and expansion, subculturing and passaging.
View our Primary Stem Cell Isolation and Expansion Portfolio
-
Compared to primary stem cells, iPSCs have abundant sources, higher passaging numbers, pluripotency, and avoid ethical concerns associated with the use of embryonic stem cells. iPSC generation is a complex process that reprograms adult somatic cells into a pluripotent, embryonic stem cell-like stage.
View our iPSC Generation Portfolio

-
Stem cells comprise a diverse population with varying properties and potential. Characterization testing helps classify stem cells based on their differentiation potential, self-renewal capacity, and other markers.
View our Characteristic Testing Portfolio
-
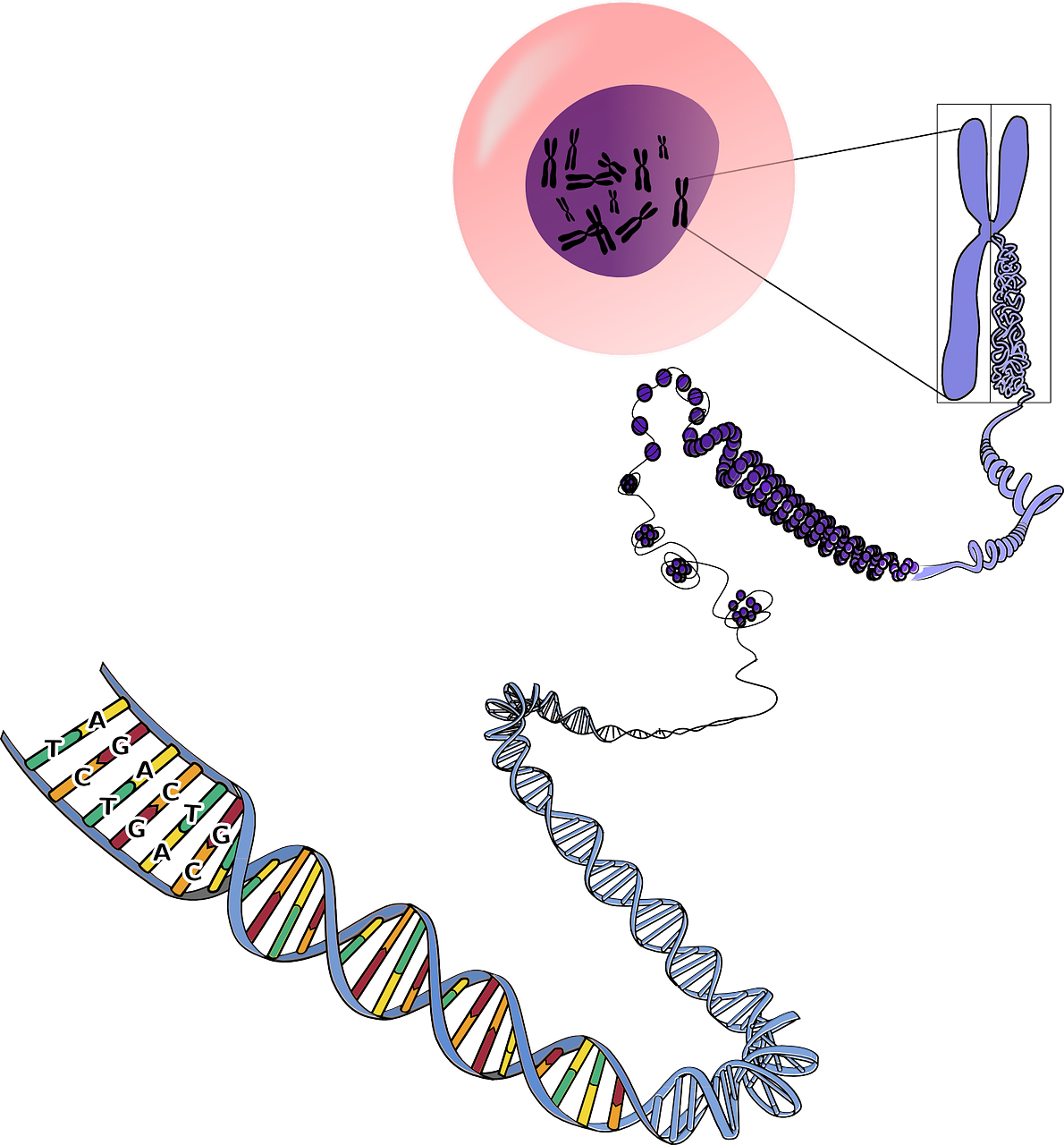
Gene modification of stem cells provides valuable tools for understanding gene function, modeling diseases, developing therapies, and advancing personalized medicine. It has the potential to revolutionize the field of regenerative medicine and contribute to improved treatments for a wide range of genetic disorders and other diseases.
View our Gene Modifying of Stem Cells Portfolio
-
Animal models provide a platform for evaluating the efficacy and safety of stem cell treatments in vivo. They allow researchers to assess the safety of stem cell transplantation, including the potential risks of immune rejection, tumor formation, and other adverse effects. Our company has various mature animal models involving over one hundred types of diseases.
View our Animal Models Portfolio

-
In vitro and in vivo pharmacology studies are crucial for assessing the safety, efficacy, and mechanistic understanding of stem cell therapies. They contribute to the development and optimization of stem cell-based treatments, ensuring their safety and increasing the likelihood of successful translation from preclinical research to clinical practice.
View our In vitro and In vivo Pharmacology Portfolio
-
Exosomes are small (~50‐150 nm) extracellular vesicles (EVs) released from various cell types, including stem cells. AcceGen provide Exosome Isolation & Quantification, Exosome Characterization & Identification, Exosome Engineering, Exosomes Labeling & Tracking, Exosome Multiomics Analysis, Functional Studies of Exosomes service.
For more information, please click Stem Cell-derived Exosomes
-
Through controlled manipulation of signaling molecules and environmental cues, scientists can guide stem cells to differentiate into other cells or organoid along desired pathways. Its potential to revolutionize regenerative medicine, disease modeling, and personalized therapies is immense.
View our Stem Cell Differentiation Portfolio