Stem Cell-derived Exosomes
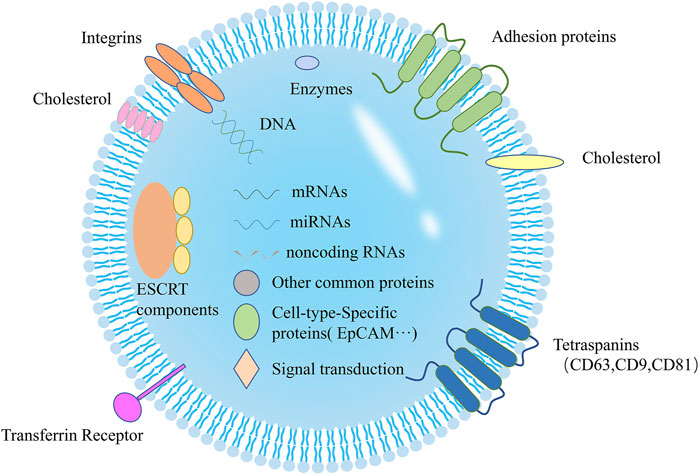
Exosomes are small (~50‐150 nm) extracellular vesicles (EVs) released from various cell types and found in body fluids and cell culture supernatants. Exosomes have been proposed as a mechanism for facilitating the intercellular exchange of macromolecules. They enable the transfer of proteins, lipids, mRNA, miRNA, and DNA between cells, thereby contributing to intercellular communication in various biological processes. These include apoptosis, antigen presentation, angiogenesis, inflammation, coagulation, and specifically, the modulation of the cancer microenvironment and the immune response. In addition, exosomes can be readily obtained from body fluids, and their composition may directly reflect the physiological and/or pathological state of the patient. Consequently, the isolation and characterization of exosomes from body fluids can provide highly valuable information for early detection, disease monitoring, and the development of effective treatments against cancer and autoimmune diseases.
Exosome Isolation & Quantification
- Ultracentrifugation: Based on density and size under centrifugal force is generally considered being the gold standard method for extracellular vesicles isolation.
- Precipitation: Of the common techniques used to isolate exosomes, precipitation offers high yield and the most straightforward workflows. While the specificity is intermediate for the difficulty separating exosomes from protein aggregates, lipoproteins, and viruses.
- Immunomagnetic bead method: Compared to ultracentrifugation, immunomagnetic bead method can be used for qualitative and quantitative determination of exosomes. It has strong specificity, high sensitivity, high purity and high yield, and does not set the upper limit of the starting sample volume when based on magnetic beads. What’s more, this method can guarantee the morphological integrity of exosomes.
- Size Exclusion Column (SEC): Probably the most suitable method, alone or in combination, to isolate exosomes in particular from plasma or serum, since it allows the separation of exosomes from important contaminants such as proteins and HDL. To optimally use SEC for dilute matrices, such as urine or cell culture media, first these samples must be concentrated by an ultracentrifugation or ultrafiltration step.
- Microfluidics (MF): Microfluidics (MF) technology uses chips with specific antibody-mediated binding to capture exosomes. Microfluidics platforms have many advantages, including enhanced reliability, sensitivity, accessibility, lower consumption of samples and reagents, quicker processing and response times, and the possibility of automated multiplexing. With a microfluidics platform, we can provide high throughput isolation and purification services for our customers.
Exosome Characterization and Identification
The exosome characterization is specific and identical, so several characterization indexes are needed to determine whether the extracted components are exosomes. Using the following techniques or methods, we can characterize and identify exosome, including size distribution, surface markers, protein composition, and more.
Exosome Isolation & Quantification
- Transmission electron microscope (TEM)
- Scanning electron microscope (SEM)
- Nanoparticle tracking analysis (NTA)
- Western blot (WB)
- Enzyme-linked immunosorbent assay (ELISA)
- Flow cytometry
- Fluorescence-activated cell sorting (FACS).
- Multiplexing bead arrays.
- Gene expression analysis (mRNA and microRNA).
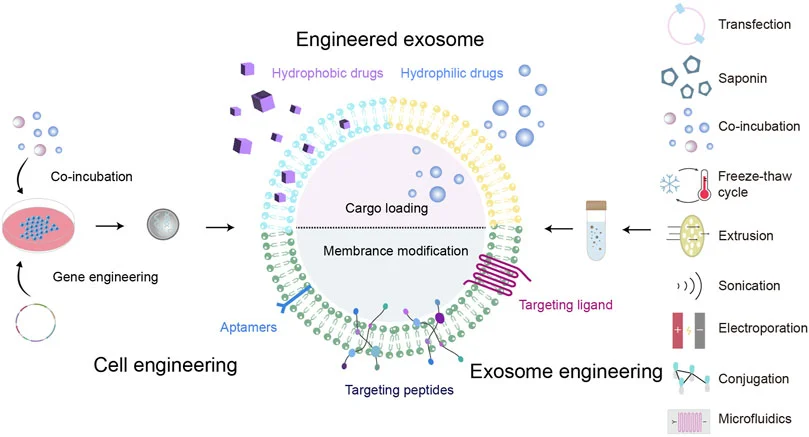
Exosome engineering
Natural exosomes may have problems such as weak targeting and susceptible to be quickly cleared in the body, resulting in poor treatment effect. Therefore, they are usually modified to form engineered exosomes. The method of gene editing uses genetically modified parent cells as the main strategy to integrate the therapeutic agent into the corresponding exosomes, and is suitable for RNA or proteins that cannot be directly loaded onto exosomes.
Categories:
- Cargo loading
- Engineered exosome production
- Surface Modification
Exosomes labeling and tracking
- Exosome fluorescent labeling
- Fluorescence exosome purification
- Fluorescent exosome concentration labeling
Exosome multiomics analysis
Exosome proteomics analysis: Including analysis of exosomal protein expression patterns, analysis of exosomal protein function models, and analysis of exosomal protein post-translational modifications.
Exosome metabolomics analysis: Including differential metabolite screening and qualitative/quantitative analysis of target metabolites/metabolic pathways.
Exosome lipidomics analysis: Including exosome lipid composition and level analysis, differential expression analysis of exosomal lipid molecules, qualitative/quantitative analysis of targeted lipid molecules.
Functional Studies
AcceGen assists in conducting functional studies of exosome, including cell-to-cell communication, signal transduction, immune modulation, targeted drug delivery, cell proliferation and disease diagnosis & treatment.
Disease Diagnosis
Exosomes are rich in biomarkers for disease diagnosis and prognosis. They are mainly applied in cancer and have also made some progress in the fields of cardiovascular diseases, tuberculosis and central nervous system diseases.
Methods:
Liquid biopsy
Biomarkers (miRNA, protein, RNA and cDNA) detection
Disease Treatment Through Exosome-Targeted Drug Delivery System
Categories:
Delivery of genetic drugs
Delivery of traditional Chinese medicine
Delivery of western medicine
Methods:
Pre-secretory Drug Loading: Inculding gene editing and Co-incubation with progenitor cells
Post-secretory Drug Loading: Co-incubation with exosomes, electroporation, sonication, extrusion, freeze-thaw cycle and saponin-assisted treatment.
Surface Modification: Including chemical linking of targeting peptides, modification of exosomal membranes or progenitor cells by genetic engineering, magnetic nanoparticle technology, electrostatic interaction and post-insertion.