Lentivirus Packaging Services
The lentiviral system is very effective at delivering genetic material to whole model organisms and almost all mammalian cells, including non-dividing, inactive or growing, and difficult-to-transfect cells such as neuron, primary and stem cells. Unlike plasmid DNA vector that allows only transient and episomal expression of foreign genes in the host cell, the lentiviral vector can achieve permanent expression through integration into the host cell genome.
At AcceGen, We provide a comprehensive selection of lentiviral constructs, libraries, or other lenti-constructs in the form of ready-to-transduce, pre-packaged, VSV-g pseudotyped lentiviral particles. Our highly experienced scientists are ready to tackle difficult cloning projects, including those involving large or toxic clones, and clones with high GC content or highly repetitive sequences.
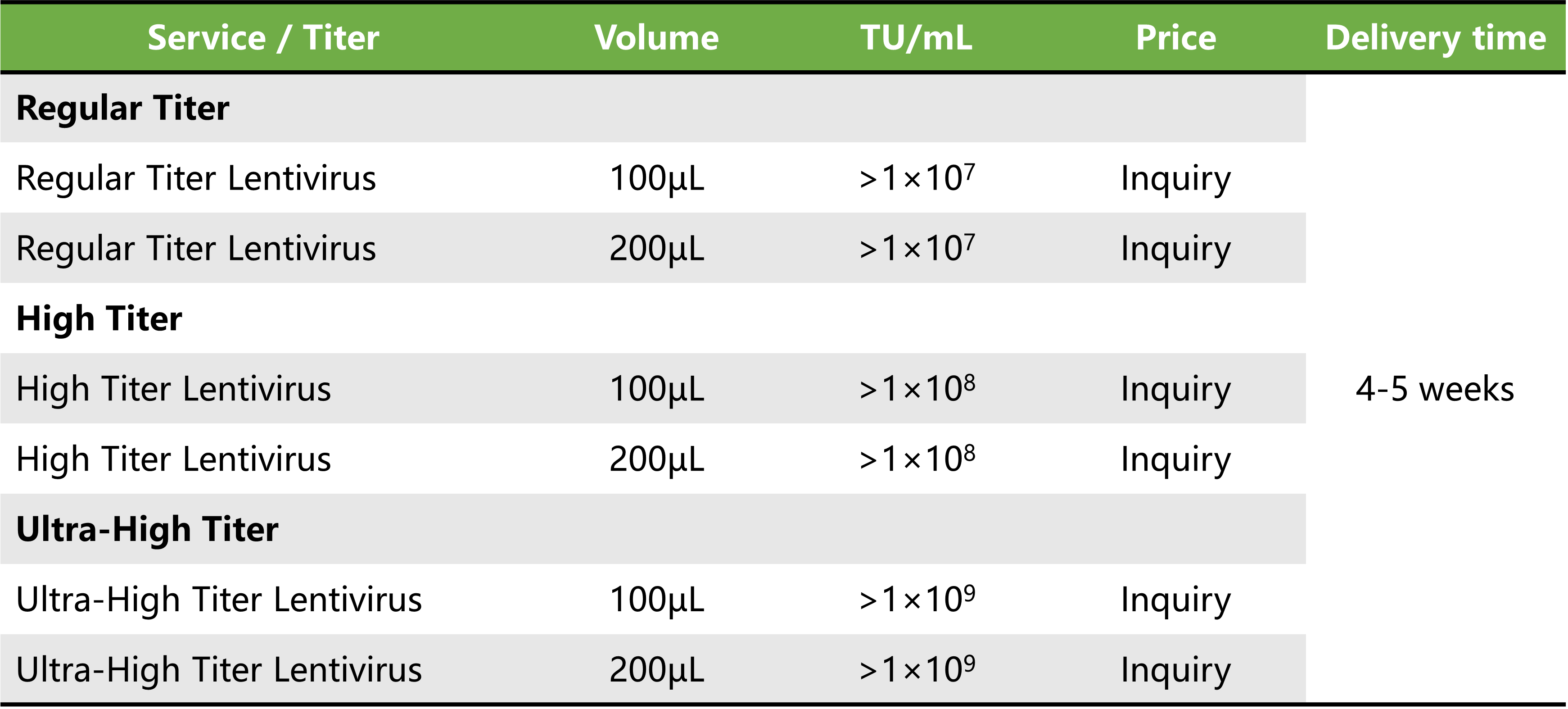
Service Details
Price and delivery time
Shipping and storage
Our lentivirus is carefully stored in HBSS buffer and shipped to you on dry ice to ensure its stability and quality. Upon receiving the lentivirus, it should be stored at -80°C for long-term storage (stable for at least 6 months) or -20°C for use within one week. The shelf life of our lentivirus is approximately one year. Please avoid repeated freeze-thaw cycles of lentivirus. By following these storage guidelines, you can ensure the optimal performance and longevity of the lentivirus.
Lentivirus production and quality control (QC)
We co-transfect the transfer plasmid containing the gene of interest (GOI) along with our exclusive envelope plasmid encoding VSV-G, and packaging plasmids encoding Gag/Pol and Rev, into HEK293T packaging cells as the third-generation lentivirus packaging system.
To ensure the production of ultra-purified lentivirus suitable for in vivo applications, we subject viral particles to additional purification steps. These steps include sucrose cushion ultracentrifugation, which helps remove impurities, and concentration through ultrafiltration, which further enhances the concentration of viral particles.
The titer of the lentivirus is assessed using RT-qPCR with primers that target the long-inverted terminal repeat (LTR) found in the viral genome. By comparing the results to a standard curve generated from a plasmid sample with a known concentration, we are able to accurately quantify the titer of lentivirus. As part of our rigorous quality control measurements, each recombinant lentivirus undergoes several assessments. This includes titer measurement to determine its concentration, sterility testing to ensure the absence of bacteria and fungi, and mycoplasma detection to confirm its purity. If the transfer vector encodes a fluorescent protein, we perform a transduction test to detect the corresponding fluorescence, validating its functionality.
Advantages:
- High viral titer: Our lentiviral vector can be packaged into high titers (>108 TU/ml), enabling efficient transduction of mammalian cells.
- Reagent precipitation: Minimize contaminants in viral preps for impr oved purity.
- Fast turnaround time: Experience prompt delivery of your lentiviral vector preparations.
- Broad tropism: With the VSV-G envelope protein, our vector can transduce cells from various species and cell types, including neurons.
- Customizable promoter: Our vector allows for the insertion of user-defined promoters, offering flexibility in gene expression.
- In vitro and in vivo effectiveness: Our vector is effective for transduction in cultured cells as well as live animals.
- Safety: Our lentiviral vector system incorporates safety measures, minimizing the risk of replication-competent viruses.
Overall, our lentiviral vector system provides permanent integration, high titer, broad tropism, customizable promoters, uniform delivery, and safety, making it a valuable gene delivery tool.