Adenovirus Packaging Services
Adenoviral vector is a widely studied viral vector used in cell and gene therapy applications. It demonstrates exceptional efficiency in transducing various mammalian cell types and remains as episomal DNA without integrating into the host genome. Adenoviral vectors are preferred for in vivo gene delivery due to their high transduction efficiency and short-term gene expression. They are used in gene therapy and vaccination, holding great promise for the treatment of genetic disorders and cancers.
AcceGen is your trusted provider of comprehensive adenovirus services tailored for cell and gene therapy developments. Our services include all aspects of adenovirus production, ensuring high-quality vectors with superior titer, purity, viability, and consistency. With our expertise in recombinant adenovirus production, vector cloning, and packaging services, we aids researchers and clinicians in developing effective gene therapies and vaccines. Our advanced technologies and reagents contribute to successful adenovirus-based gene delivery, facilitating the advancement of the field of cell and gene therapy.
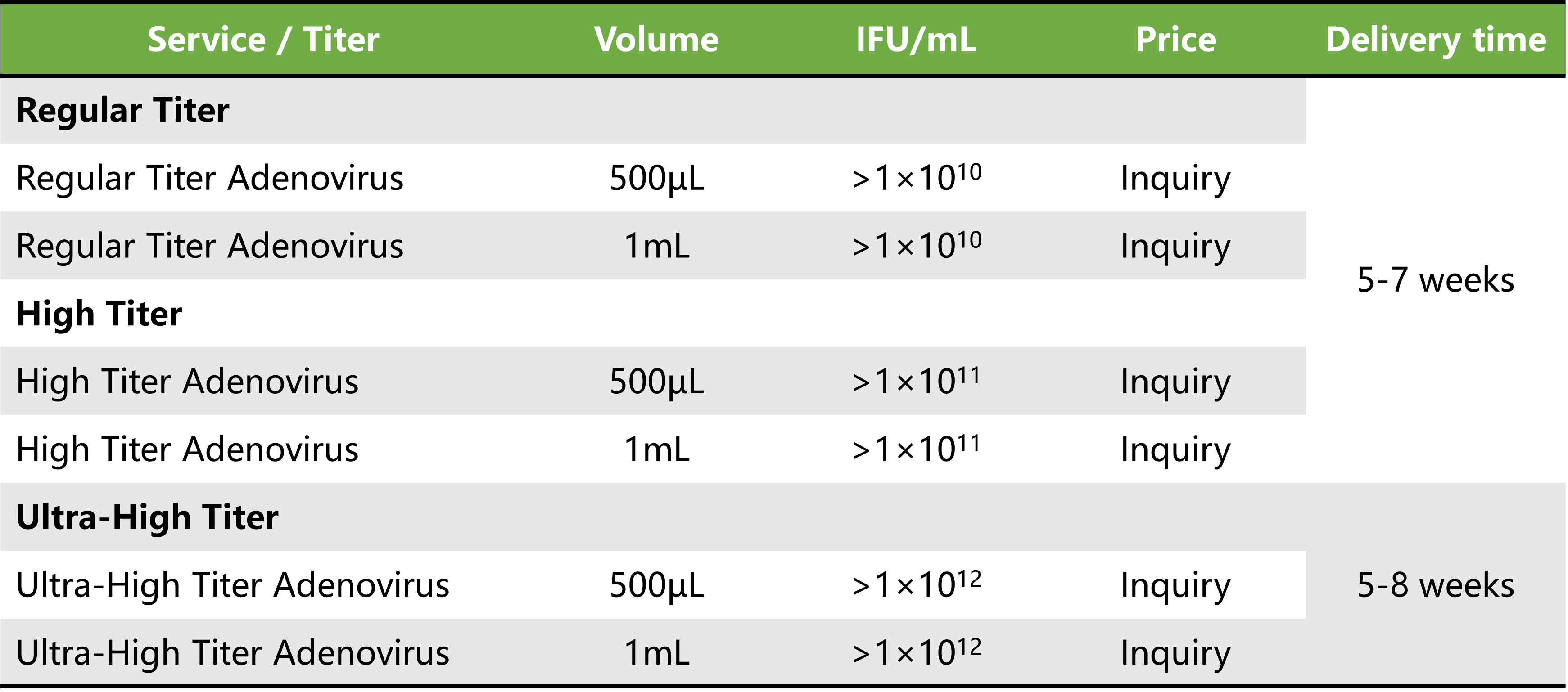
Service Details
Price and delivery time
Shipping and storage
Our non-ultra-High adenovirus is stored in HBSS buffer and ultra-High adenovirus is stored in GTS buffer. Adenovirus product is shipped on dry ice and should be stored at -80°C for long-term storage (stable for at least 1 year) or -20°C for short-term storage (2-3 weeks). While adenovirus is more stable than many other viruses, it is advisable to minimize freeze-thaw cycles to maintain optimal virus activity.
Adenovirus production and quality control (QC)
Our adenovirus manufacturing process involves linearizing the vector, transfecting it into packaging cells, and collecting recombinant adenovirus particles. Ultra-purified adenovirus undergoes further purification for improved quality and concentration using cesium chloride (CsCl) gradient ultracentrifugation.
To ensure the highest quality of our adenovirus products, we implement comprehensive quality control(QC) measures. These measures include titer measurement, sterility testing, mycoplasma detection, and optional endotoxin assay. By performing these rigorous quality control tests, we maintain the highest standards of our adenovirus products. If you have any specific additional quality control requirements, we are also able to offer tailored QC services upon request, further ensuring the quality and suitability of our adenovirus products for your specific research or therapeutic development.
Advantages:
- Efficient production: Our manufacturing process efficiently generates recombinant adenovirus particles, ensuring high yields for experimental and therapeutic applications.
- Ultra-purification option: We offer ultra-purified adenovirus, which undergoes additional purification steps for enhanced quality and concentration.
- Comprehensive quality control: Our rigorous quality control measures ensure the integrity, purity, and sterility of our adenovirus products.
- Customizable services: We provide tailored solutions to meet specific research and therapeutic needs, including additional QC services upon request.
- Reliable support: AcceGen offers expert technical assistance and prompt customer support to guide customers through their adenovirus-related projects.
Overall, AcceGen’s adenovirus services provide efficient production, ultra-purification options, comprehensive quality control, customizable solutions, and reliable support, making them ideal for a wide range of research applications.