Adeno-Associated Virus (AAV) Packaging Service
AAV plays a crucial role in cell and gene therapy applications. It is effective in transducing various mammalian cell types, making it valuable for delivering therapeutic genes into target cells. One of the key advantages of AAV is low immunogenicity and nonpathogenic nature, which contribute to its excellent safety profile. This safety aspect is essential for the successful gene therapy interventions. AAV vectors have the capacity to achieve long-term expression of therapeutic genes in host cells, offering potential treatments for genetic disorders, neurodegenerative diseases, and cancer.
AcceGen’s Custom AAV Service offers comprehensive support for your specific needs, including the design, cloning, and viral packaging of your custom AAV construct. Leveraging our extensive expertise in AAV technology, we have developed proprietary technologies and reagents that optimize production protocols for recombinant AAV. This results in improved titer, purity, potency, and consistency, particularly for the AAV vector systems employed in our vector cloning services.
Service Details
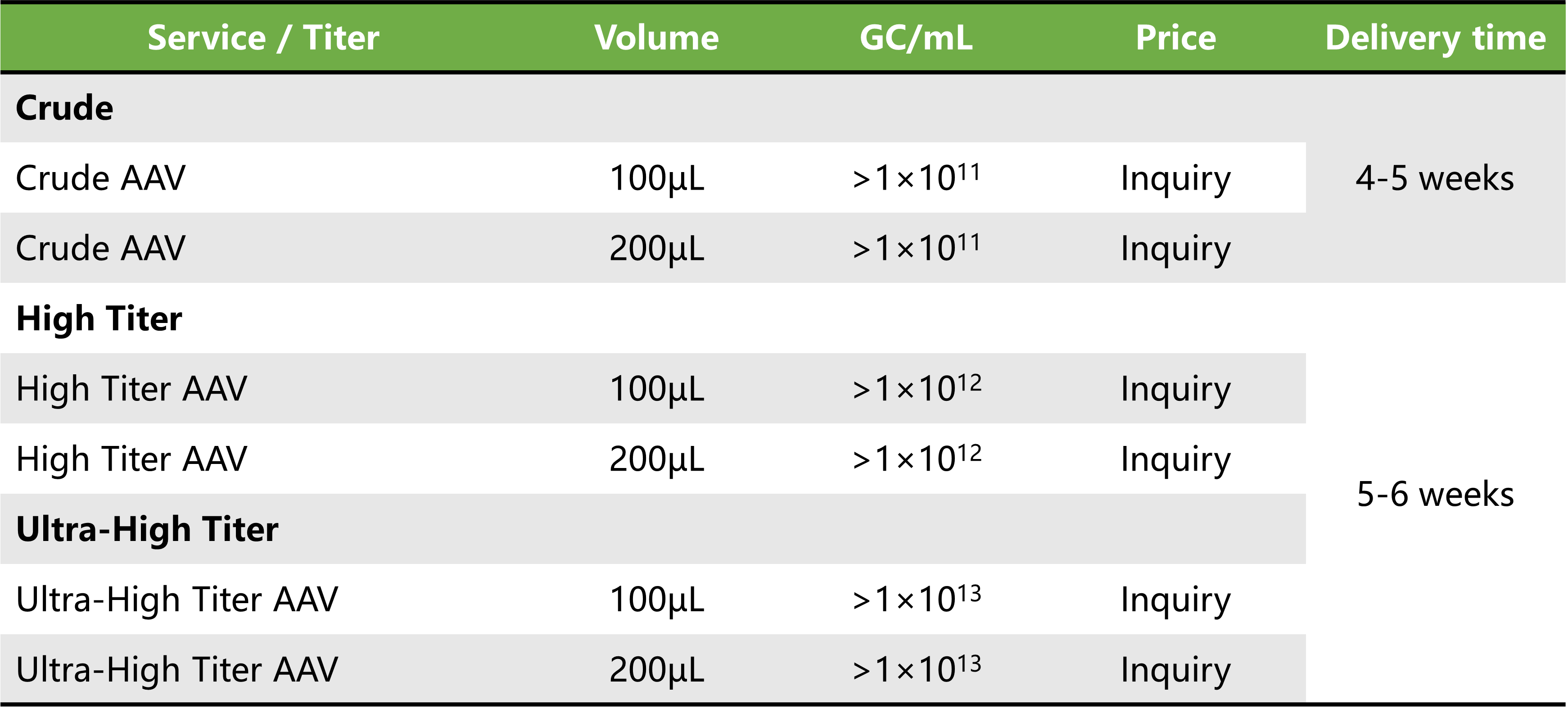
Price and delivery time
Shipping and storage
Our non-ultra-high AAV is stored in a Tris-based buffer, while our ultra-high AAV is stored in a PBS-based buffer. To ensure their stability and quality, AAV are shipped on dry ice and should be stored at -80°C for long-term storage (stable for at least 1 year) or -20°C for short-term storage (2-3 weeks). Thawed AAV can be stored at 4°C for 1-2 weeks. Please avoid repeated freeze-thaw cycles of AAV.
AAV production and quality control (QC)
In our AAV production process, we perform co-transfection of the transfer plasmid carrying the gene of interest (GOI), our proprietary Rep-cap plasmid, and a helper plasmid encoding adenovirus genes in HEK293T packaging cells. Following an incubation period, the viral particles are harvested. For the production of ultra-purified AAV, we empoly cesium chloride (CsCl) gradient ultracentrifugation as an additional purification step.
To determine the titer of AAV, we rely on qPCR analysis that targets the inverted terminal repeats (ITRs) present in the viral genome. This allows us to accurately quantify the number of genome copies in the sample. By comparing the qPCR results to a standard curve generated from a plasmid sample of known concentration, we can determine the physical titer of the AAV preparation. This approach ensures reliable and consistent measurement of AAV titer for our packaging services.
Advantages:
- Comprehensive serotype selection: We provide access to 15 different AAV serotypes, including AAV1, AAV2, AAV2-retro, AAV2-QuadYF, AAV3, AAV5, AAV6, AAV6.2, AAV7, AAV8, AAV9, AAVrh 10, AAVDJ, AAV-DJ/8 and AAV-PHP.eb. This broad range allows for tailored transduction approaches.
- Fast turnaround time: We prioritize efficiency and aim to deliver your AAV vectors within 4-6 weeks, ensuring your research progresses without delays.
- Competitive titer: We guarantee a high titer of >1×1013 GC/mL, enabling efficient and potent transduction of target cells and tissues.
- Flexibility: Our AAV services offer customizable volumes, titers, and purification options, allowing you to obtain vectors tailored to your specific experimental needs.
- Safety: AAV is considered the safest viral vector system available. It is replication-deficient and does not pose a risk of causing human diseases, providing peace of mind for researchers.
- Low risk of host genome disruption: Upon transduction, AAV vectors remain as episomal DNA in the nucleus, minimizing the risk of integration into the host genome.
- High viral titer: Our AAV vectors can be packaged into high-titer viruses. Through our virus packaging service, you can achieve titers exceeding >1013 genome copies per ml (GC/ml), ensuring robust transduction efficiency.
Overall, our AAV services provide customizable options, fast turnaround times, and high safety. With 15 serotypes available, AAV vectors have broad tropism and a low risk of host genome disruption. They are effective in both in vitro and in vivo applications, making them versatile research tools.