- In-Stock Tumor Cell Lines
- Human Orbital Fibroblasts
- Human Microglia
- Human Pulmonary Alveolar Epithelial Cells
- Human Colonic Fibroblasts
- Human Type II Alveolar Epithelial Cells
- Human Valvular Interstitial Cells
- Human Thyroid Epithelial Cells
- C57BL/6 Mouse Dermal Fibroblasts
- Human Alveolar Macrophages
- Human Dermal Fibroblasts, Adult
- Human Lung Fibroblasts, Adult
- Human Retinal Muller Cells
- Human Articular Chondrocytes
- Human Retinal Pigment Epithelial Cells
- Human Pancreatic Islets of Langerhans Cells
- Human Kidney Podocyte Cells
- Human Renal Proximal Tubule Cells
The Origin of Vero Cells
Vero cells are recognized by the World Health Organization (WHO) and Chinese Pharmacopoeia in producing vaccines. Vero cells, also known as African green monkey kidney cells, were officially isolated from African green monkey kidneys by Japanese scholars Yasumura Y and Kawakita Y in 1962[1]. It was the first aneuploidy attachment-dependent cell used in producing human biological products and establishing cell lines and cell bank of different generations at the same time.
The Relationship between Vero Cells and Viral Vaccines
Viral vaccines have been widely used in preventing many diseases, including rabies, influenza, and smallpox[2]. However, vaccines are still not affordable for vulnerable populations in endemic areas, the cost of the vaccine and the tremendous need worldwide are the main hurdles for equitable and global use of human viral vaccines[3].
The Vero cells can be applied to vaccine production ranges from live rotavirus vaccines to live attenuated influenza vaccines to the development of inactivated whole virus vaccines. Vero cells cannot express antiviral protein interferon due to their inherent genetic defects. Therefore, the broad susceptibility of Vero cells has promoted the development of vaccine production using Vero cells as a substrate.
Vero cells have the following advantages compared with the primary and diploid cell lines for producing biological products. Firstly, the cell bank is easy to establish and preserve, at the same time it can be continuously passaged with a fast growth rate. Secondly, Vero cells have stable genetic traits and a low probability of malignancy. Thirdly, Vero cells are sensitive to a variety of viruses and have high virus titers. Finally, Vero cells could exceedingly resistant to various cultural conditions, and the requirements are not high that it can grow well on the surface of the microcarrier, which proves that Vero cells have significant value in the development of a series of viral vaccines[4].
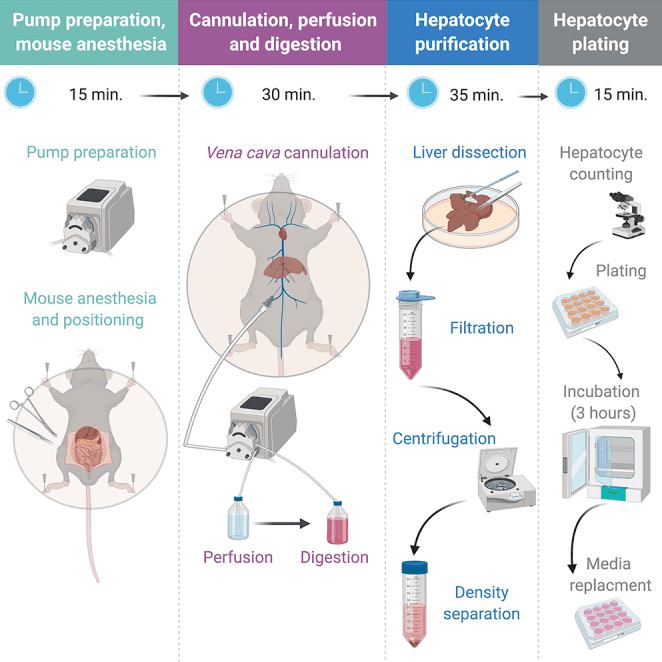
How to Culture adherent Vero Cells
The growth of Vero cells is anchorage-dependent, and Vero cells can only proliferate when provided with a suitable surface[5]. Adherent Vero cells were cultured separately in MEM, Medium 199, and DMEM supplemented with 10% FBS under a 5% CO2 and 37℃ cultures. The researchers found that Vero cells, the highest cell concentration reached 25.6 ± 1.1 × 104 cells/cm2 in DMEM + FBS group, which increased 50.1% compared to the MEM+FBS group and 89.6% of the M199+FBS group (figure1) [6].
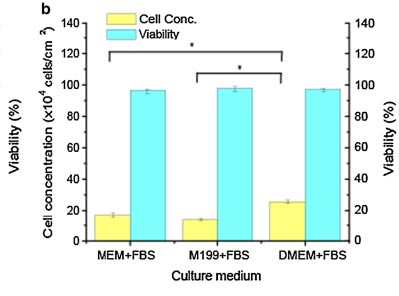
Figure1. Comparison of Vero cell growth in different culture media (n=5)[6]
Besides, more spheroidal dead cells were found in the M199 + FBS medium group. In conclusion, the DMEM + FBS medium group is more suitable for the growth of Vero cells and was ultimately selected for subsequent research (figuer2).
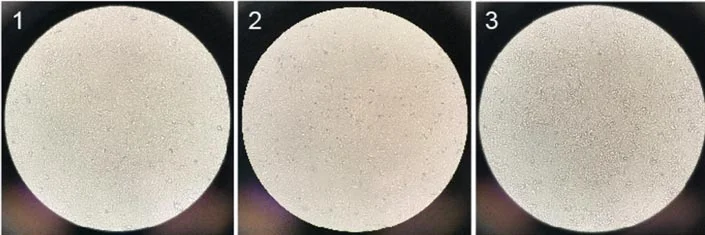
Figure2. Vero cell growth pictures in different media on day 4, (1) MEM+FBS, (2) M199+FBS, (3) DMEM+FBS [6]
FBS can provide Vero cells with the necessary substances for attachment culture in vitro, such as attachment factors, growth factors, and hormones, which could provide a stable environment in vitro and promote high-density cell growth.
However, the serum is a complex mixture of elements, and its components are not completely clear. Therefore, the use of serum in cell culture technology has many disadvantages, which increased the instability of the vaccine production process and the difficulty of vaccine quality control. Researchers have begun to use animal cell serum-free media to replace traditional bovine serum media, which has become an essential development trend for viral vaccine production.
Adaptation of adherent Vero cells to suspension growth in IPT-AFM
Application: The use of a new suspension Vero cell line combined with IPT-AFM, an in-house developed animal-component-free medium will highly contribute to reducing the vaccine cost.
Adherent Vero Cells were initially grown either in Minimum Essential Medium (MEM) supplemented with 10% FBS or IPT-AFM (an in-house developed animal component-free medium). Cell culture was used as a baffle shake flask at a seeding density of 7×104 cells / cm2, then Cells were infected at a MOI of 0.1, without proceeding to a medium exchange.
For suspension culture, five commercial serum-free/chemically defined media were tested next to IPT-AFM. Vero cells were using three different methods (figure3), then cells were monitored daily among kinetic parameters. For viability assessment, cells were stained with trypan blue (0.2% (w/v) in phosphate-buffered saline (PBS) [7]. Their results show that suspended Vero cells were growing best in the third culture, as shown in figure4, and Vero cells were able to grow in suspension in IPT-AFM.
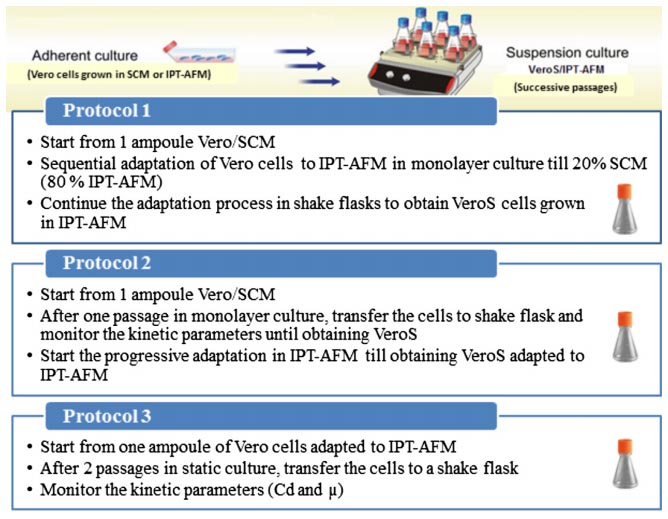
Figure3. Adaptation of adherent Vero cells to suspension culture according to the three protocols[7].
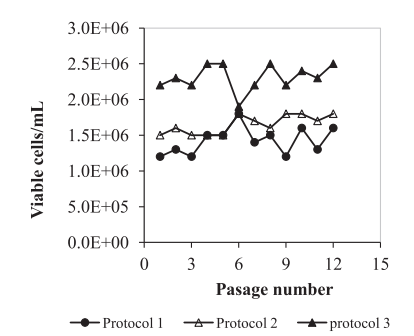
Figure4. Growth of Vero cells adapted to suspension culture in shake flasks according to 3 different protocols (data from 12 passages).
Conclusion
The main application area of Vero cell culture medium technology was the development and production of viral vaccines. Making vaccines using Vero cells as a matrix has improved the safety and controllability of product quality.
In the future, Vero cells may be used in the development and application of current coronavirus vaccines (COVID-19). COVID-19 virus may learn from the experience of developing rabies virus vaccines using Vero cells, which made Vero cells transform the adherent state into suspension culture (serum-free culture). The infection efficiency and virus titer were detected after transfecting with COVID-19 viruses.
AcceGen Vero Cell Line
AcceGen cultures and provides the African green monkey kidney cell line — Vero, Vero (AC-free), Vero 76. For more detailed information, please visit our website or contact [email protected]
References
1. Yasumura Y KY: Studies on SV40 in tissue culture-preliminary step for cancer research in vitro[J]. Nihon rinsho 1963, 21: 1201-1215.
2. Barrett PN, Terpening SJ, Snow D, Cobb RR, Kistner O: Vero cell technology for rapid development of inactivated whole virus vaccines for emerging viral diseases. Expert Review of Vaccines 2017, 16(9):883-894.
3. Khaled Trabelsi MBZ, Héla Kallel: Purification of rabies virus produced in Vero cells grown in serum free medium. vaccine 2019.
4. Shen CF, Guilbault C, Li X, Elahi SM, Ansorge S, Kamen A, Gilbert R: Development of suspension adapted Vero cell culture process technology for production of viral vaccines. Vaccine 2019, 37(47):6996-7002.
5. Orr-Burks N, Murray J, Wu W, Kirkwood CD, Todd KV, Jones L, Bakre A, Wang H, Jiang B, Tripp RA: Gene-edited vero cells as rotavirus vaccine substrates. Vaccine: X 2019, 3:100045.
6. Yang J, Guertin P, Jia G, Lv Z, Yang H, Ju D: Large-scale microcarrier culture of HEK293T cells and Vero cells in single-use bioreactors. AMB Express 2019, 9(1):70.
7. Samia Rourou MBZ, Héla Kallel: Adaptation of Vero cells to suspension growth for rabies virus production in different serum free media. Vaccine 2019.

Copyright - Unless otherwise stated all contents of this website are AcceGen™ All Rights Reserved – Full details of the use of materials on this site please refer to AcceGen Editorial Policy – Guest Posts are welcome, by submitting a guest post to AcceGen you are agree to the AcceGen Guest Post Agreement – Any concerns please contact [email protected]