- In-Stock Tumor Cell Lines
- Human Orbital Fibroblasts
- Human Microglia
- Human Pulmonary Alveolar Epithelial Cells
- Human Colonic Fibroblasts
- Human Type II Alveolar Epithelial Cells
- Human Valvular Interstitial Cells
- Human Thyroid Epithelial Cells
- C57BL/6 Mouse Dermal Fibroblasts
- Human Alveolar Macrophages
- Human Dermal Fibroblasts, Adult
- Human Lung Fibroblasts, Adult
- Human Retinal Muller Cells
- Human Articular Chondrocytes
- Human Retinal Pigment Epithelial Cells
- Human Pancreatic Islets of Langerhans Cells
- Human Kidney Podocyte Cells
- Human Renal Proximal Tubule Cells
What are Schwann cells?
Schwann Cells (SCs) are axon sheath cells that naturally exist in the peripheral nervous system (PNS) of all vertebrate species [1]. Schwann cells were mainly found in the spinal cord, although they were also present in the forebrain, brain stem, and cerebellum [2].
Formation of mature Schwann cells from ventrally migrating neural crest cells occurs through several intermediate cell populations during embryonic development, including the Schwann cell precursors found in peripheral nerves between embryonic day 12-13 and immature Schwann cells which were found between day13-15[3]. As shown in Figure 1, a schematic diagram of the main cell types involved in the formation of mature Schwann cells[4].
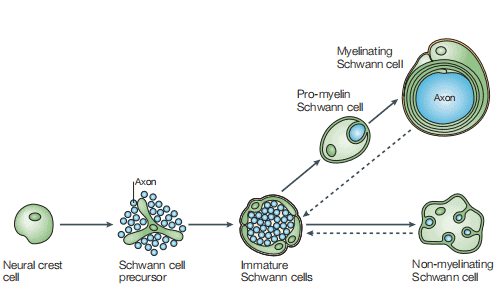
Figure 1 The Schwann cell lineage [4]
The Biological Characteristics of Schwann Cells
Schwann cells (SCs) are mainly responsible for the regeneration and repair of the peripheral nervous system (PNS) [5]. A key feature of Schwann cell biology is that their differentiation into myelin and non-myelin cells is not terminal but reversible. SCs are one of the few known cell types in adult mammals. They reprogram their genotype through dedifferentiation, self-renewal and transformation into repair cells that can promote nerve growth to respond to injury [6].
Schwann cells (SCs) have long been recognized for their ability to support repair and promote axon regeneration after peripheral nervous system injuries[7]. SCs dedifferentiation is a form of somatic cell reprogramming which gives PNS a unique self-repair ability [8]. Recent studies have also revealed that SCs are involved in tumor genesis and promoted the crosstalk between cancer cells and nerves. Therefore, researchers can further study diseases such as neuroprotection after trauma or disease by isolating Schwann cells and transplanting them to the desired site[9].
How to Isolate Schwann Cells from Rat?
It is well known that mouse Schwann cells are difficult to culture for several days because of their reduced cell division and inherently difficult to myelinate in vitro. Thus, rat Schwann cells are more commonly used[10]. Meanwhile, the cultivation of adult neurogenic SC is possible because the dissected nerve fragments or the SC in the ganglion can survive well after being separated from the body[11]. Here we take the separation of Schwann cells from rats as an example to illustrate.
The isolation steps here are referred to the study of Elisabetta Babetto and Huff et al.: Firstly, chop the sciatic nerves from rats in pieces as small as possible with a sterile blade and incubate nerves in vitro for 10 days in DMEM medium containing 10% heat-inactivated FBS and allow them to degenerate.
Secondly, degenerated nerve explants were dissociated using a 0.25% dispase/0.05% collagenase solution and stop the enzyme activity by adding 2.5 mL 40% FBS in HBSS[10].
Thirdly, transfer the solution containing the small pieces of tissue to a tube and centrifuge at 300g for 10 min and resuspend the pellet in 10% FBS in DMEM. Crush the tissues through the P1000 tip approximately 35 times until there are no visible tissue fragments and remove undissolved tissues by placing a 70μm nylon cell suppressor on a 50mL centrifuge tube.
Finally, centrifuge the cells at 300g for 10 min and place the resulting cell suspension on poly-l-lysine (PLL)-coated dishes. Purified primary Schwann cells are expanded up to passage one in DMEM media containing 10nM neuregulin (recombinant here gulin-β1) and 2μM forskolin. Experiments are performed on Schwann cells between passage 3 and passage 6 and plated on glass coated in PLL and laminin from Engelbreth-Holm-Swarm murine sarcoma basement membrane. Schwann cells are grown for experiments in DMEM supplemented with 10% FBS and without neuregulin or forskolin [12].
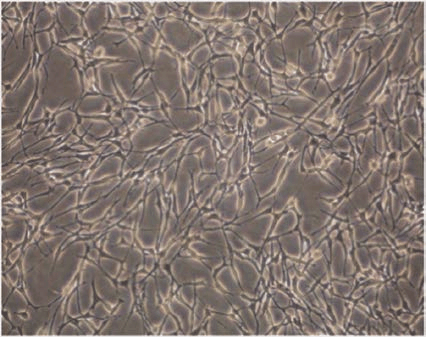
Figure 2 The morphology of cultured Schwann cells[13]
Applications of Schwann cells
Recently, gene therapy targeted at myelin Schwann cells in PNS which has played a positive role in the possibility of replacing or silencing genes related to hereditary demyelinating neuropathy [14]. Naruse found that the supernatant of cultured dental pulp stem cells increased the proliferation and production of myelin-related proteins in Schwann cells, indicating that Schwann cells are the main target of diabetic neuropathy cell transplantation and the potency of Schwann cells as a target of cell therapy for diabetic neuropathy will be demonstrated[15].
Conclusion:
The successful isolation and culture of primary Schwann cells can enable researchers to gain insight into the characteristics of Schwann cells and their mechanisms in disease. Meanwhile, primary Schwann cells will provide an excellent research model for the treatment of related diseases. Therefore, they are likely to promote SCs biological and therapeutic research in many years to come.
Where to Get Schwann Cells for research?
The Schwann cells provide a relatively simple, well-defined and accessible mammalian model for the study of a number of developmental questions. AcceGen provides the most authentic Human Schwann Cells, Rat Schwann Cells, CD-1 Mouse Schwann Cells and CD57 Mouse Schwann Cells. Besides, a wide range of cell products from nervous system for research use are also available in AcceGen. All the products of AcceGen are strictly comply with international standards. For more detailed information, please visit our product portfolio or contact inquiry@accegen.com.
Reference:
1. Gao Z, Min C, Xie H, Qin J, He X, Zhou S: TNFR2 knockdown triggers apoptosis-induced proliferation in primarily cultured Schwann cells. Neurosci Res 2020, 150:29-36.
2. Garcia-Diaz B, Baron-Van Evercooren A: Schwann cells: Rescuers of central demyelination. Glia 2020, 68(10):1945-1956.
3. Furlan A, Adameyko I: Schwann cell precursor: a neural crest cell in disguise? Dev Biol 2018, 444 Suppl 1:S25-S35.
4. Jessen KR, Mirsky R: The origin and development of glial cells in peripheral nerves. Nat Rev Neurosci 2005, 6(9):671-682.
5. Kim HS, Kim JY, Song CL, Jeong JE, Cho YS: Directly induced human Schwann cell precursors as a valuable source of Schwann cells. Stem Cell Res Ther 2020, 11(1):257.
6. Jessen KR, Mirsky R, Lloyd AC: Schwann Cells: Development and Role in Nerve Repair. Cold Spring Harb Perspect Biol 2015, 7(7):a020487.
7. Bray ER, Cheret J, Yosipovitch G, Paus R: Schwann cells as underestimated, major players in human skin physiology and pathology. Exp Dermatol 2020, 29(1):93-101.
8. Jessen KR, Mirsky R: Negative regulation of myelination: relevance for development, injury, and demyelinating disease. Glia 2008, 56(14):1552-1565.
9. Ferdoushi A, Li X, Jamaluddin MFB, Hondermarck H: Proteomic Profile of Human Schwann Cells. Proteomics 2020, 20(1):e1900294.
10. Babetto E: A Schwann Cell–Neuron Coculture System to Study Neuron–Glia Interaction During Axon Degeneration, vol. 2143; 2020.
11. Jessen KR, Arthur-Farraj P: Repair Schwann cell update: Adaptive reprogramming, EMT, and stemness in regenerating nerves. Glia 2019, 67(3):421-437.
12. Huff TC, Sant DW, Camarena V, Van Booven D, Andrade NS, Mustafi S, Monje PV, Wang G: Vitamin C regulates Schwann cell myelination by promoting DNA demethylation of pro-myelinating genes. J Neurochem 2020.
13. Xu S, Bao W, Men X, Liu Y, Sun J, Li J, Liu H, Cai H, Zhang W, Lou J et al: Interleukin-10 Protects Schwann Cells against Advanced Glycation End Products-Induced Apoptosis via NF-kappaB Suppression. Exp Clin Endocrinol Diabetes 2020, 128(2):89-96.
14. Sargiannidou I, Kagiava A, Kleopa KA: Gene therapy approaches targeting Schwann cells for demyelinating neuropathies. Brain Res 2020, 1728:146572.
15. Naruse K: Schwann Cells as Crucial Players in Diabetic Neuropath. Myelin 2019:345-356.

Copyright - Unless otherwise stated all contents of this website are AcceGen™ All Rights Reserved – Full details of the use of materials on this site please refer to AcceGen Editorial Policy – Guest Posts are welcome, by submitting a guest post to AcceGen you are agree to the AcceGen Guest Post Agreement – Any concerns please contact marketing@accegen.com