- In-Stock Tumor Cell Lines
- Human Orbital Fibroblasts
- Human Microglia
- Human Pulmonary Alveolar Epithelial Cells
- Human Colonic Fibroblasts
- Human Type II Alveolar Epithelial Cells
- Human Valvular Interstitial Cells
- Human Thyroid Epithelial Cells
- C57BL/6 Mouse Dermal Fibroblasts
- Human Alveolar Macrophages
- Human Dermal Fibroblasts, Adult
- Human Lung Fibroblasts, Adult
- Human Retinal Muller Cells
- Human Articular Chondrocytes
- Human Retinal Pigment Epithelial Cells
- Human Pancreatic Islets of Langerhans Cells
- Human Kidney Podocyte Cells
- Human Renal Proximal Tubule Cells
Background: Skeletal Muscle Development and Beef Cattle Breeding
Skeletal muscle is a vital organ in vertebrates and is part of the muscular system, whose main function is to convert energy into strength and heat through contraction and to engage in certain endocrine activities that contribute to overall health[1; 2; 3]. Skeletal muscle energy metabolism and muscle fiber phenotype are regulated by several essential signaling and metabolic factors, including peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α), peroxisome proliferator-activated receptors (PPARs), and hypoxia-inducible factor 1-alpha (Hif-1α). These factors are intricately associated with mitochondrial function, as well as the regulation of sugar and lipid metabolism[4]. (Figure.1) In the context of beef cattle farming, the development of skeletal muscle emerges as a critical determinant. It exerts a substantial impact on both the overall health of cattle and the ultimate quality of beef production. This connection between skeletal muscle development and beef cattle breeding underscores the importance of understanding and managing skeletal muscle physiology for optimal outcomes in the industry[5].
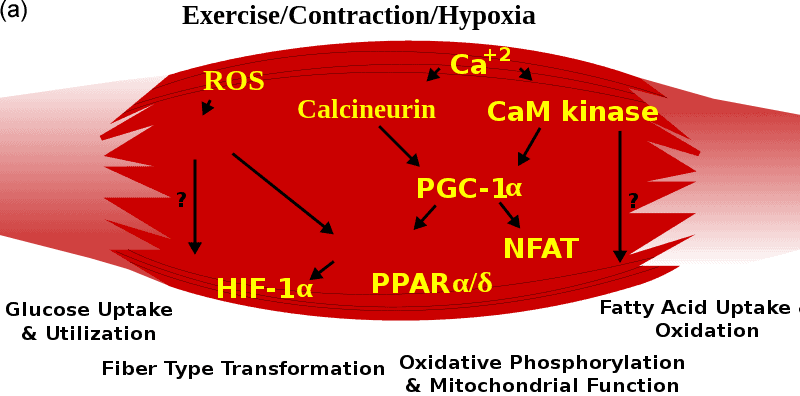
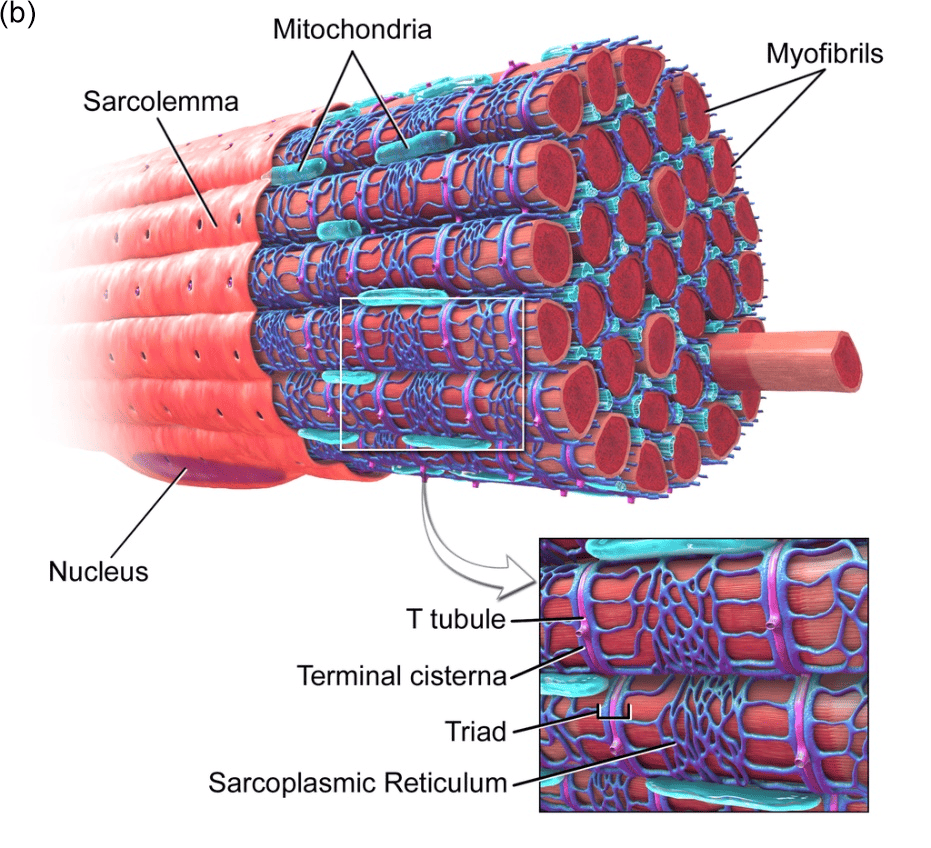
Figure.1 Skeletal Muscle. (a) Signal regulation in skeletal muscle[6]. (b) Skeletal muscle 3D structure[7].
Bovine Muscle Satellite (Myosatellite) Cells and Culture Protocol
Myosatellite cells (also known as muscle stem cells) are small multipotent cells in the skeletal muscle, which are precursors to skeletal muscle cells and bear the responsibility of stem cell self-renewal in skeletal muscle organization[8]. Myosatellite cells, pivotal in skeletal muscle development and homeostasis maintenance, have garnered a focus of attention in studies related to beef cattle breeding. Research on the mechanisms of myosatellite cell and skeletal muscle development has the potential to provide scientific strategies for improving the quality and yield of the beef production. Furthermore, in alignment with evolving concepts related to animal and environmental protection, cultured meat derived on bovine skeletal muscle cells and myosatellite cells has emerged as a burgeoning research focus. These highlight the value of bovine myosatellite cell applications[9].
Bovine myosatellite cells are mainly obtained by primary extraction, and a concise description of the isolation, extraction, and culture protocol is provided below[10]. Bovine semimembranosus muscle (which can also be extracted from other skeletal muscle tissues) is obtained by dissection immediately following slaughter, ensuring stringent aseptic conditions and removal of connective tissue and visible fat. Subsequently, the muscle tissue was ground and subjected to digestion in EBSS solution containing 1% Pronase for 1 h. The bovine muscle statellite cells are then isolated through differential centrifugation. The cells are cultured in DMEM with 10% FBS and 1% antibiotic-antimycotic at 37℃ with 5% CO2, and additional medium with 10% DMSO is employed while cryopreservation. In vitro, mature bovine skeletal muscle cells are obtained from bovine myosatellite cells, which serve as precursor cells for induced differentiation. The differentiation medium commonly involves the addition of insulin, dexamethasone, and pantothenic acid, and alternative differentiation protocols exist that circumvent the above ingredients and utilize selegiline instead.
Application: Bovine Muscle Satellite Cells in the Publications
Research on the application of bovine myosatellite cells has focused on two main directions. The first direction centers on the field of beef cattle breeding, the study of bovine skeletal muscle development and metabolism in order to pinpoint potential strategies for enhancing bovine skeletal muscle growth and achieving phenotypically superior breeds through fetal programming. The second direction is to explore research related to artificially cultured meat.
In the field of beef cattle breeding, researchers commonly aim to identify crucial targets capable of promoting the proliferation and differentiation of bovine myosatellite cells to promote the development of skeletal muscle and then increase beef cattle growth, or to regulate the lipid metabolism of skeletal muscle to produce beef with enhanced flavor and desirable marbling. These studies typically validate potential targets regulating skeletal muscle development and lipid metabolism by using primary derived bovine myosatellite cells as an in vitro model. For example, Zhang et al. found that unsaturated fatty acid palmitoleic acid (POA) can promote lipogenic but not myogenic differentiation of bovine myosatellite cells, which is a potential method to increase the fat content of beef skeletal muscle to improve the taste and quality of beef[5]. In an antecedent publication by the same research group, they found that fatty acids facilitate the differentiation of bovine skeletal muscle satellite cells through ELOVL3[11]. In addition, in another publication, this group demonstrate that activation of peroxisome proliferator-activated receptor gamma (PPARγ) by ciglitazone directly affects the expression levels of adipose-related genes in bovine muscle satellite cells[10]. These findings reveal a potential pathway to improve beef quality through PPARs that regulate myogenic or lipogenic differentiation of bovine muscle satellite cells. Additonally, numerous other factors have been identified to regulate the differentiation of bovine myosatellite cells, such as transcription elongation factor A3 (TCEA3)[12], miR-377[13], Vitamin A[14], and early growth response 1 (EGR1)[15].
Cultured meat, a relatively nascent field in recent years, regards bovine muscle satellite cells as a pivotal raw material. In this context, Messmer et al. verified the feasibility of a medium devoid of animal-derived components for meat culture by RNA sequencing and other methodologies, and demonstrated that a serum-free differentiation medium effectively supports the differentiation of bovine myosatellite cells, thus paving the way for beef culture[16]. Furthermore, Andreassen et al. explored the use of food-grade microcarriers derived from by-products as potential tools to enhance the differentiation of bovine myosatellite cells thereby contributing to the prospects of cultured meat production[17].
Conclusion
Meat constitutes a fundamental part of the human daily diet, and it provides a plentiful source of essential nutrients including proteins, lipids, vitamins, metallic trace elements, and more. Therefore, it is imperative for the beef cattle industry and the emerging field of cultured meat, to explore methogologies that facilitate the production of sufficient quantities of high-quality meat products. Within this context, the study of bovine skeletal muscle and myosatellite cells, which wield a significant impact on beef yield and quality, is also of great potential value.
Where to Get Animal Primary Cells for Your Research?
AcceGen isolates and offers a wide range of high-quality animal primary cells, such as Bovine Muscle Satellite Cells, Pig Muscle Satellite Cells, C57BL/6 Mouse Astrocytes, and Rat Liver Kupffer Cells. These cell products provide you with a convenient means to research. To get more information, please refer to: Animal Primary Cells.
It is our pleasure to help relative researches to move forward. All the products of AcceGen are strictly comply with international standards. For more detailed information, please visit our product portfolio or contact inquiry@accegen.com.
References
[1] J.G.Y. Betts, Kelly A.; Wise, James A.; Johnson, Eddie; Poe, Brandon; Kruse, Dean H.; Korol, Oksana; Johnson, Jody E.; Womble, Mark; Desaix, Peter, “Interactions of Skeletal Muscles, Their Fascicle Arrangement, and Their Lever Systems”. Interactions of skeletal muscles. OpenStax. (2013).
[2] “Structure of Skeletal Muscle | SEER Training”. training.seer.cancer.gov.
[3] L. Grube, R. Dellen, F. Kruse, H. Schwender, K. Stühler, and G. Poschmann, Mining the Secretome of C2C12 Muscle Cells: Data Dependent Experimental Approach To Analyze Protein Secretion Using Label-Free Quantification and Peptide Based Analysis. J Proteome Res 17 (2018) 879-890.
[4] X. Chen, Y. Ji, R. Liu, X. Zhu, K. Wang, X. Yang, B. Liu, Z. Gao, Y. Huang, Y. Shen, H. Liu, and H. Sun, Mitochondrial dysfunction: roles in skeletal muscle atrophy. J Transl Med 21 (2023) 503.
[5] J. Zhang, Q. Li, K.M.C. Nogoy, J. Sun, B. Sun, Y. Wang, L. Tang, J. Yu, X. Jin, X. Li, and S.H. Choi, Effect of palmitoleic acid on the differentiation of bovine skeletal muscle satellite cells. J Anim Sci Technol 63 (2021) 919-933.
[6] https://en.wikipedia.org/wiki/Skeletal_muscle#/media/File:Muscle_pathways.svg.
[7] Blausen.com staff (2014). “Medical gallery of Blausen Medical 2014”. WikiJournal of Medicine 1 (2). DOI:10.15347/wjm/2014.010. ISSN 2002-4436.
[8] F. Kadi, N. Charifi, C. Denis, J. Lexell, J.L. Andersen, P. Schjerling, S. Olsen, and M. Kjaer, The behaviour of satellite cells in response to exercise: what have we learned from human studies? Pflugers Arch 451 (2005) 319-27.
[9] V. Bodiou, P. Moutsatsou, and M.J. Post, Microcarriers for Upscaling Cultured Meat Production. Front Nutr 7 (2020) 10.
[10] J. Zhang, Q. Li, Y. Yan, B. Sun, Y. Wang, L. Tang, E. Wang, J. Yu, K.M.C. Nogoy, X. Li, and S.H. Choi, Effect of ciglitazone on adipogenic transdifferentiation of bovine skeletal muscle satellite cells. J Anim Sci Technol 63 (2021) 934-953.
[11] J. Xu, D. Liu, H. Yin, H. Tong, S. Li, and Y. Yan, Fatty acids promote bovine skeletal muscle satellite cell differentiation by regulating ELOVL3 expression. Cell Tissue Res 373 (2018) 499-508.
[12] Y. Zhu, H.-L. Tong, S.-F. Li, and Y.-Q. Yan, Effect of TCEA3 on the differentiation of bovine skeletal muscle satellite cells. Biochemical and Biophysical Research Communications 484 (2017) 827-832.
[13] Y. Zhu, P. Li, X. Dan, X. Kang, Y. Ma, and Y. Shi, miR-377 Inhibits Proliferation and Differentiation of Bovine Skeletal Muscle Satellite Cells by Targeting FHL2. Genes (Basel) 13 (2022).
[14] B. Wang, W. Nie, X. Fu, J.M. de Avila, Y. Ma, M.J. Zhu, M. Maquivar, S.M. Parish, J.R. Busboom, M.L. Nelson, and M. Du, Neonatal vitamin A injection promotes cattle muscle growth and increases oxidative muscle fibers. J Anim Sci Biotechnol 9 (2018) 82.
[15] W. Zhang, H. Tong, Z. Zhang, S. Shao, D. Liu, S. Li, and Y. Yan, Transcription factor EGR1 promotes differentiation of bovine skeletal muscle satellite cells by regulating MyoG gene expression. J Cell Physiol 233 (2018) 350-362.
[16] T. Messmer, I. Klevernic, C. Furquim, E. Ovchinnikova, A. Dogan, H. Cruz, M.J. Post, and J.E. Flack, A serum-free media formulation for cultured meat production supports bovine satellite cell differentiation in the absence of serum starvation. Nat Food 3 (2022) 74-85.
[17] R.C. Andreassen, S.B. Rønning, N.T. Solberg, K.G. Grønlien, K.A. Kristoffersen, V. Høst, S.O. Kolset, and M.E. Pedersen, Production of food-grade microcarriers based on by-products from the food industry to facilitate the expansion of bovine skeletal muscle satellite cells for cultured meat production. Biomaterials 286 (2022) 121602.

Copyright - Unless otherwise stated all contents of this website are AcceGen™ All Rights Reserved – Full details of the use of materials on this site please refer to AcceGen Editorial Policy – Guest Posts are welcome, by submitting a guest post to AcceGen you are agree to the AcceGen Guest Post Agreement – Any concerns please contact marketing@accegen.com