- In-Stock Tumor Cell Lines
- Human Orbital Fibroblasts
- Human Microglia
- Human Pulmonary Alveolar Epithelial Cells
- Human Colonic Fibroblasts
- Human Type II Alveolar Epithelial Cells
- Human Valvular Interstitial Cells
- Human Thyroid Epithelial Cells
- C57BL/6 Mouse Dermal Fibroblasts
- Human Alveolar Macrophages
- Human Dermal Fibroblasts, Adult
- Human Lung Fibroblasts, Adult
- Human Retinal Muller Cells
- Human Articular Chondrocytes
- Human Retinal Pigment Epithelial Cells
- Human Pancreatic Islets of Langerhans Cells
- Human Kidney Podocyte Cells
- Human Renal Proximal Tubule Cells
Alveolar Epithelium Cell and Type I / II Epithelial Cells
Alveolar epithelium cell (AEC) is an essential structure involved in the pathogenesis and rehabilitation of acute lung injury, which was divided into type I and type II[1]. The majority of type I and type II cells arise from progenitors[2]. Type I epithelial cells form alveolar walls cover 80% of the alveolar surface area for gas exchange. Type II epithelial cells cover 5% of the alveolar surface area. Its principal function is to secrete alveolar surfactants and regulate the balance of epithelial fluid, including the production of components such as pathogens interact and trigger immune cell activation[3].
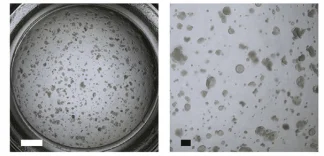
Figure1. Image of a representative well from passaged AEC2 cells[4].
The Difference between Type I and Type II Cells
Alveolar type 2 epithelial cells are facultative progenitors in the adult lung and respond to injury or loss by self-renewal and differentiation to alveolar type 1 epithelial cells [5]. The initial damage to the alveolar epithelium usually occurs on type I cells, which are more susceptible to injury toward type II cells and has limited repair capabilities. If the epithelial lightly damaged, alveolar type 2 epithelial cells (AEC2s) will spread and reproduce to cover the alveolar surface and restore the function of the lungs without Fibrosis[6].
Recently, it has been shown that mechanical forces promote the alveolar type I epithelial cells (AT1) differentiation, whereas AT2 progenitors are shielded from these forces by a niche cell. The mechanical forces may help explain how AT1 and AT2 cells migrate into and intermix within the mature alveolus[2]. ATII cells readily differentiate into ATI cells when cultured submerged.
Idiopathic Pulmonary Fibrosis (IPF)
Idiopathic pulmonary fibrosis (IPF) is a persistent and ultimately fatal fibrotic interstitial lung disease of unknown cause. Meanwhile, patients have a median survival of 3-5 years[7]. It usually occurs in males and people who are older than 50 years old. Patients usually experience symptoms, such as difficulty breathing, coughing and fever[8]. IPF is associated with damage to alveolar type 2 epithelial cells (AEC2s) with significant changes in phenotype and function[9]. For example, AEC2s release changes in various fibrotic media, such as transforming growth factor (TGF) -β, IL-1 β or WNT ligand[10]. Therefore, alveolar type 2 epithelial cells were targeted administration that may be a promising treatment strategy for IPF.
How to Successfully Separate and Purify Alveolar Type 2 Epithelial Cells?
The first step in the isolation process is to minify the distal lung tissue and then wash it to remove residual connective tissue, blood cells, and blood vessels.
Then the tissue is digested and filtered through a filter. After doing the above preparations, use of a three-phase Percoll gradient (4%, 10%, and 30%) to extract alveolar type 2 epithelial cells by centrifugation (ATII cells at the interface of the 10% and 30% phases).
Finally, the cell pellet is resuspended and used to establish cultures and determine phenotype by immunofluorescent staining of ATII cell markers[11, 12]. Flow-marked AEC2s specific biomarkers EPCAM and HT2-280[4] could be used to confirm whether the aspirated cells are AEC2s.
How to Culture Alveolar Type 2 Epithelial Cells?
Seed 2.5 × 105 or 7.5 × 105 isolated cells into T25 or T75 tissue culture flasks that may be cultured in SAGM and grow in a humidified incubator with a 5% CO2 atmosphere. Monitor for fungal or bacterial growth under a light microscope. To maintain high density, passage ATII cells up to 1:4 when they reach 75-80% confluence (Splits greater than 1:4 are not recommended)[11].
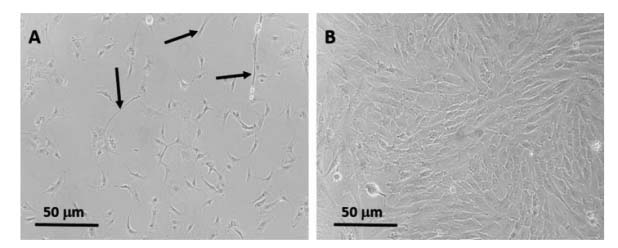
Figure2. Fibroblast removal from ATII cultures. Fibroblasts are notable for their elongated spindle-like morphology (A). Following their removal, ATII cells exhibit a cobblestone morphology when allowed to grow to confluence (B)[11].
Drug Reveals the Effect on IPF Through Action on ATII Cells
To date, Pirfenidone and Nintedanib are the only approved drugs known to decelerate IPF disease progression. Mareike investigated the effects of Nintedanib and pirfenidone on alveolar epithelial cells and then observed the outcome of drugs on pulmonary fibrosis. They treated primary murine and human alveolar epithelial type II (pmATII) cells with Pirfenidone or Nintedanib. Murine ATII cells were derived from a single dose of bleomycin (2 U/kg body weight) model of fibrosis , and qPCR, Western blotting, Immunofluorescent staining, and ELISA are applied to determine the function of epithelial and mesenchymal cells[9]. They found that Nintedanib and Pirfenidone have both been shown to exhibit anti-fibrotic capacities in animal models of lung fibrosis in vivo. Meanwhile, CHOP was activated by AP-1 and c-Ets-1 that played a pivotal role in AECII maladaptive ER stress responses and consecutive fibrosis, which may offer new therapeutic prospects in IPF[13].
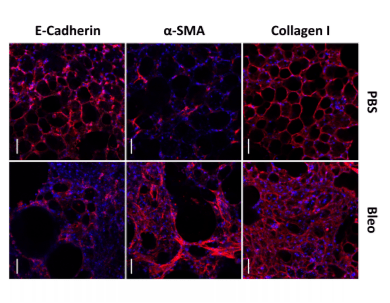
Figure3. Representative immunofluorescence analysis of Collagen I, α-SMA and E-Cadherin in control (PBS), and fibrotic (Bleo) 3D-LTCs after 48 h in culture[9].
Conclusion
IPF is a disease that severely affects the structure and function of the lungs, and its occurrence and development are very complicated. AT Ⅱ cell, as a essential cell to maintain the structure and function of the lung, which is extremely critical in the occurrence and development of pulmonary fibrosis. A large number of researches have proved the important role of AT Ⅱ in IPF. Soon the treatment of IPF will go to a higher level with continuous exploration of its development mechanism.
AcceGen Human Respiratory System Primary Cells
AcceGen provides high-quality Human Respiratory System Primary Cells, including Human Pulmonary Alveolar Epithelial Cells (HPAEpiC), Human Type II Alveolar Epithelial Cells, Human Lung Fibroblasts, Human Pulmonary Fibroblasts, Human Lung Cells (uncultured), etc., to support your IPF and other respiratory-related diseases research.
We also provide Animal Respiratory-related Primary Cells, and Human & Animal Lung Cancer Cell Lines, for more detailed information, please visit our product portfolio or contact inquiry@accegen.com.
References
1. Seiji Y, Ito T, Nakamura Y, Nakaishi-Fukuchi Y, Matsuo A, Sato N, Nogawa H: Alveolus-like organoid from isolated tip epithelium of embryonic mouse lung. Human Cell 2019, 32(2):103-113.
2. David B. Franka b, c,1, Ian J. Penkalad, Jarod A. Zeppb,e, Aravind Sivakumara,b,c, Ricardo Linares-Saldanac,d,e, William J. Zachariasf, Katharine G. Stolza, Josh Pankina,b, MinQi Lua, Qiaohong Wangc,d,e, Apoorva Babuc,e,LiLic, Su Zhouc, Michael P. Morleyb,c,e, Rajan Jainc,d,e,1, and Edward E. Morriseyb,c,d,e,1: Early lineage specification defines alveolar epithelial ontogeny in the murine lung. PNAS 2019.
3. Rackley CR, Stripp BR: Building and maintaining the epithelium of the lung. J Clin Invest 2012, 122(8):2724-2730.
4. Shiraishi K, Nakajima T, Shichino S, Deshimaru S, Matsushima K, Ueha S: In vitro expansion of endogenous human alveolar epithelial type II cells in fibroblast-free spheroid culture. Biochem Biophys Res Commun 2019, 515(4):579-585.
5. Sitaraman S, Na CL, Yang L, Filuta A, Bridges JP, Weaver TE: Proteasome dysfunction in alveolar type 2 epithelial cells is associated with acute respiratory distress syndrome. Sci Rep 2019, 9(1):12509.
6. Kelly A. Correll KEE, Rachel L. Zemans*,Elizabeth F. Redente,Karina A. Serban,Douglas Curran-Everett,Robert J. Mason: Transitional human alveolar type II epithelial cells suppress extracellular matrix and growth factor gene expression in lung fibroblasts. Physiology 2019.
7. Pleasants R, Tighe RM: Management of Idiopathic Pulmonary Fibrosis. Ann Pharmacother 2019, 53(12):1238-1248.
8. Sheng G, Chen P, Wei Y, Yue H, Chu J, Zhao J, Wang Y, Zhang W, Zhang H-L: Viral Infection Increases the Risk of Idiopathic Pulmonary Fibrosis. Chest 2019.
9. Lehmann M, Buhl L, Alsafadi HN, Klee S, Hermann S, Mutze K, Ota C, Lindner M, Behr J, Hilgendorff A et al: Differential effects of Nintedanib and Pirfenidone on lung alveolar epithelial cell function in ex vivo murine and human lung tissue cultures of pulmonary fibrosis. Respir Res 2018, 19(1):175.
10. Katsura H, Kobayashi Y, Tata PR, Hogan BLM: IL-1 and TNFalpha Contribute to the Inflammatory Niche to Enhance Alveolar Regeneration. Stem Cell Reports 2019, 12(4):657-666.
11. Lee DF, Chambers MA: Isolation of Alveolar Type II Cells from Adult Bovine Lung. Curr Protoc Toxicol 2019, 80(1):e71.
12. Vukosavljevic B, Hittinger M, Hachmeister H, Pilger C, Murgia X, Gepp MM, Gentile L, Huwer H, Schneider‐Daum N, Huser T et al: Vibrational spectroscopic imaging and live cell video microscopy for studying differentiation of primary human alveolar epithelial cells. Journal of Biophotonics 2019, 12(6):e201800052.
13. Klymenko O, Huehn M, Wilhelm J, Wasnick R, Shalashova I, Ruppert C, Henneke I, Hezel S, Guenther K, Mahavadi P et al: Regulation and role of the ER stress transcription factor CHOP in alveolar epithelial type-II cells. J Mol Med (Berl) 2019, 97(7):973-990.

Copyright - Unless otherwise stated all contents of this website are AcceGen™ All Rights Reserved – Full details of the use of materials on this site please refer to AcceGen Editorial Policy – Guest Posts are welcome, by submitting a guest post to AcceGen you are agree to the AcceGen Guest Post Agreement – Any concerns please contact marketing@accegen.com