- In-Stock Tumor Cell Lines
- Human Orbital Fibroblasts
- Human Microglia
- Human Pulmonary Alveolar Epithelial Cells
- Human Colonic Fibroblasts
- Human Type II Alveolar Epithelial Cells
- Human Valvular Interstitial Cells
- Human Thyroid Epithelial Cells
- C57BL/6 Mouse Dermal Fibroblasts
- Human Alveolar Macrophages
- Human Dermal Fibroblasts, Adult
- Human Lung Fibroblasts, Adult
- Human Retinal Muller Cells
- Human Articular Chondrocytes
- Human Retinal Pigment Epithelial Cells
- Human Pancreatic Islets of Langerhans Cells
- Human Kidney Podocyte Cells
- Human Renal Proximal Tubule Cells
Introduction: Mesenchymal Stem Cells
Mesenchymal stem cells (MSCs), also known as mesenchymal stromal cells, are an important member of the stem cell family. Mesenchymal stem cells are adult stem cells that are found not only in bone marrow, but also in skeletal muscle, the outer bone membrane, and bone trabeculae. Mesenchymal stem cells originate in the mesoderm in early development and have been isolated from various sources such as liver, placenta, muscle, umbilical cord, etc[1]. MSCs are pluripotent stem cells with self-replicating capability[2, 3]. Mesenchymal stem cells differentiate into hematopoietic cells, stromal cells, muscle cells, osteoblasts, chondrocytes, liver cells, and other cells in an appropriate environment. Mesenchymal stem cells can differentiate into a variety of tissues with great clinical application value.
Culture Protocol
Complete medium and differentiation medium are used in the traditional MSCs culture. Currently, MSCs for clinical application are mainly derived from the bone marrow and umbilical cord. Umbilical cord mesenchymal stem cells (UC-MSCs)gradually replace bone marrow-derived MSCs for easier obtain. Here we describe the separation method of bone marrow-derived MSCs. Take appropriate amounts of fresh tissue aspirate collected under sterile conditions. Filter the cell suspension with a 70 mm strainer to remove any cell clumps. Then cells were precipitated at 500 grams in a bench centrifuge for 5 minutes. The cells were cultured in 10 ml complete MSC medium (SCM015 or SCM045) at a density of 25×106 cells /ml in a T75 dish. The plates were incubated in an incubator at 37℃ and 5% CO2. After 3 hours, the medium was replaced with 10 ml of fresh complete medium to remove the non-adherent cells. Once the cells have reached about 80% confluence, the cells can be subcultured.[4] Subsequently, immunofluorescence staining and flow cytometry are operated to guarantee the species and purity of MSC cells. They were characterized by the expression of CD105, CD73, and CD90, but not CD45, CD34, CD14 or CD11b, CD79a, CD19, or HLA-DR. Placing MSCs in a medium containing specific supplements, for the adipogenesis process they are mainly dexamethasone, indomethacin, insulin and isobutyl methylxanthine, for chondrogenesis cell culture in DMEM medium supplemented with insulin, transferrin, selenium, linoleic acid, selenium acid, pyruvate, ascorbic phosphate, dexamethasone and TGF-βIII, which may additionally be assisted by the addition of IGF-1 and BMP-2 (BMP; bone morphogenetic proteins). In turn, osteogenesis is induced by the presence of ascorbic acid,β-glycerophosphate and dexamethasone.
Application of Mesenchymal Stem Cells
Mesenchymal stem cells show a variety of biological functions, such as multidirectional differentiation, immunomodulation, angiogenesis, anti-apoptosis, anti-fibrosis and tissue repair. Under specific conditions, MSCs can differentiate into nerve cells, osteoblasts, chondrocytes, muscle cells and adipocytes, which play a regulatory role in hematopoiesis, immune inflammatory response, angiogenesis and other important functions of the human body. Therefore, mesenchymal stem cells are widely used in the treatment of many diseases, including a variety of autoimmune diseases, graft-versus-host diseases, hematological diseases, vascular diseases, diabetes, tissue or organ damage and degenerative diseases.
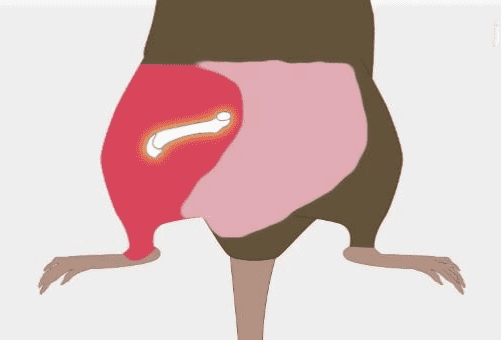
Mesenchymal stem cells promote tissue homeostasis or regeneration in neural, wound, kidney, liver, bone and heart (Figure.1). Mesenchymal stem cells exert the osteogenesis function by inducing Mesenchymal stem cells with vitamin D3, β-glycerophosphate, ascorbic acid, or BMP-2, BMP-4, BMP-6, and BMP-7 [5]. In addition, mesenchymal stem cells contain multiple trophic factors that induce the recovery of damaged cells. MSCs can reverse the fibrotic activity of injured tissues and decrease myofibroblasts [6]. Furthermore, these cells release pro-angiogenic factors including VEGF, IGF-1, and anti-inflammatory factors that participate in the recovery of tissue function. For instance, MSCs increase neovascularization of ischemic myocardium through VEGF in a mouse model of heart disease [7]; what’s more, IGF-1 exerts an advantageous effect on the survival and proliferation of cardiomyocytes [8].

Figure.1Effect of bone marrow mesenchymal stem cell-based regenerative medicine[9]
In addition, mesenchymal stem cells can affect the immune system through direct cell-cell contact or secretion of cytokines. Mesenchymal stem cells have immune privileges due to the low expression of MHC-I and no expression of costimulatory factors such as CD86, CD40 and CD80 or MHC-II. In the treatment of refractory systemic lupus erythematosus, MSCs have been reported to have a 4-year complete remission rate of 34%. Mesenchymal stem cells also have a remission rate of 50% to 60% for lupus nephritis. Furthermore, mesenchymal stem cells have been expanded to treat Crohn’s disease and graft-versus-host disease (GVHD).
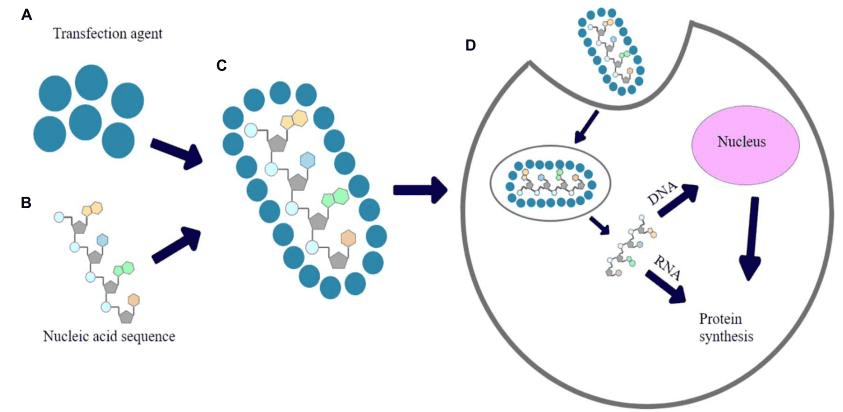
Recent studies have demonstrated that extracellular vesicles (EVs) showed a similar therapeutic effect as mesenchymal stem cells in selected animal models [10]. The main classes of EVs include exosomes, microvesicles (MVs), apoptotic bodies (ApoBDs), nanovesicles and large oncosomes. (Figure.2) Exosomes, carrying a mass of signal molecules such as RNA, DNA, proteins, and lipids, are the most important of these EV subsets[1]. MSC exosomes have been reported exert therapeutic efficacies against many diseases such as myocardial ischemia/reperfusion injury, wound healing, hepatic regeneration, and more recently cartilage and bone regeneration [11]. Intriguingly, their numerous advantages over MSCs, such as increased viability, higher uptake, lower immune response, reduced risk of embolism, and potential to cross the blood-brain barrier, have rendered exosomes a promising candidate emerging as an effective “cell-free” therapeutic approach in the field of regenerative medicine [12].

Figure.2Mechanisms of secretion of EVs.[13]
Drug delivery is an important strategy for the development of MSC technology. The scientists loaded MSCs with prodrug invertase, apoptosis inducer, or oncolytic virus to treat the tumor. These mesenchymal stem cells migrate to the tumor in response to chemical attractants, angiogenic factors, or inflammatory signals secreted by the tumor.
Mesenchymal stem cells have been genetically engineered to produce trophic cytokines or other beneficial gene products in numerous preclinical models by transfecting MSCs with viral or non-viral vectors. Over the last few decades, these MSCs have successfully been engineered to express therapeutic peptides and proteins in animal models. CRISPR/Cas9-mediated gene knockdown in MSCs has been proved effective in treating diseases such as myocardial infarction[14]. Targeted gene knock-in promoted the differentiation of mesenchymal stem cells and, in turn, ameliorated the insufficiency of functional cells in local sites[15]. Genetically modified MSCs have been evaluated for the safety, tolerability, and efficacy in treating patients with advanced gastrointestinal adenocarcinoma [16].
Recently MSCs are also used for handling the acute respiratory distress syndrome (ARDS) of COVID-19 due to their multifactorial mode of action [17].
Drug development and research became an important application field of artificial intelligence (AI) technology. The discovery of drug molecules by AI has been selected as one of the “top ten global breakthrough technologies” by MIT Technology Review in 2020. The advances of AI are expected to help identify the essential elements of MSCs and boost the understanding of MSCs therapies.
Challenges in Clinical Applications of Mesenchymal Stem Cells
So far, there are totally 10 kinds of mesenchymal stem cells approved in the world (Korea, Canada, Japan, India and Europe), however, none of them have been approved by the FDA. Ryoncil (remestemcel-L) is promising to be the first FDA-approved GVHD treatment for children younger than 12, but is still in the stage of safety verification. Factors contributing to the failure of MSCs’ clinical applications include poor quality control and inconsistent characteristics of MSCs in terms of stability, heterogeneity, differentiation, and migratory capacity across studies [18, 19].
To solve these problems, it is necessary to improve the quality control system of MSCs. New studies show that MSCs comprise multiple subsets with typical surface markers. More work is needed to define these subpopulations based on biomarkers and biological functions. The efficacy of MSCs is highly dependent on their in vivo migration capacities. However, the expression profile of chemokines in damaged tissues is usually not compatible with that of receptors on MSCs. To improve the migration rate, MSCs are genetically modified to express specific chemokine receptors [20].
Prospect
At present, mesenchymal stem cell therapies have shown great promise in many fields, including rare diseases. With the development of basic stem cell research and the continuous advancement of technology, the stem cell industry has a huge room for development. We look forward to more revolutionary breakthroughs in the field of stem cell therapy in the future to benefit human health.
Where to Get MSCs for Your Research?
AcceGen offers a wide range of high-quality human/animal stem cells, including MSCs, iPSCs, ESCs, Adult Stem Cells, and Tumor Stem Cells. These cell lines provide you with a convenient means to research. To get more information, please refer to: Stem Cells.
It is our pleasure to help relative researches to move forward. All the products of AcceGen are strictly comply with international standards. For more detailed information, please visit our product portfolio or contact [email protected].
References
[1] Zhou et al. Challenges and advances in clinical applications of mesenchymal stromal cells. J Hematol Oncol. https://doi.org/10.1186/s13045-021-01037-x.
[2] Midha S, Jain KG, Bhaskar N, Kaur A, Rawat S, Giri S, et al. Tissue-specific mesenchymal stem cell-dependent osteogenesis in highly porous chitosan-based bone analogs. Stem Cells Transl Med. 2020. https://doi. org/10.1002/sctm.19-0385.
[3] Vaananen HK. Mesenchymal stem cells. Ann Med. 2005;37(7):469–79.
[4] Masoud Soleimani, Samad Nadri. A protocol for isolation and culture of mesenchymal stem cells from mouse bone marrow. Nat Protoc. 2009; 4(1): 102-6.
[5] Friedman MS, Long MW, Hankenson KD. Osteogenic differentiation of human mesenchymal stem cells is regulated by bone morphogenetic protein-6. J Cell Biochem. 2006;98(3):538–54.
[6] Quintanilha LF, et al. Canine mesenchymal stem cells show antioxidant properties against thioacetamide-induced liver injury in vitro and in vivo. Hepatol Res. 2014;44(10):E206–17.
[7] Tang JM, et al. VEGF/SDF-1 promotes cardiac stem cell mobilization and myocardial repair in the infarcted heart. Cardiovasc Res. 2011;91(3):402–11.
[8] Troncoso R, et al. New insights into IGF-1 signaling in the heart. Trends Endocrinol Metab. 2014;25(3):128–37.
[9] Margiana et al. Clinical application of mesenchymal stem cell in regenerative medicine: a narrative review. Stem Cell Research & Therapy (2022) 13:366.
[10] Rostom DM, Attia N, Khalifa HM, Abou Nazel MW, El Sabaawy EA. The therapeutic potential of extracellular vesicles versus mesenchymal stem cells in liver damage. Tissue Eng Regen Med. 2020;17(4):537–52.
[11] Azimi M, Ghabaee M, Naser Moghadasi A, and Izad M. (2019). Altered expression of miR-326 in T cell-derived exosomes of patients with relapsing remitting multiple sclerosis. Iran. J. Allergy Asthma Immunol. 18 (1), 108–113.
[12] Palma M, Hansson L, Choudhury A, Näsman-Glaser B, Eriksson I, Adamson L, et al. (2012). Vaccination with dendritic cells loaded with tumor apoptotic bodies (Apo-DC) in patients with chronic lymphocytic leukemia: Effects of various adjuvants and definition of immune response criteria. Cancer Immunol Immunother. 61 (6), 865–879.
[13] Yu Luo, Zhihua Li, Xinxin Wang, et al. Characteristics of culture-condition stimulated exosomes or their loaded hydrogels in comparison with other extracellular vesicles or MSC lysates. Frontiers in Bioengineering and Biotechnology. DOI 10.3389/fbioe.2022.1016833.
[14] Golchin A, Shams F, Karami F. Advancing mesenchymal stem cell therapy with CRISPR/Cas9 for clinical trial studies. Adv Exp Med Biol. 2020;1247:89–100.
[15] Miwa H, Era T. Tracing the destiny of mesenchymal stem cells from embryo to adult bone marrow and white adipose tissue via Pdgfralpha expression. Development. 2018;145(2):dev155879.
[16] Von Einem JC, Guenther C, Volk HD, Grutz G, Hirsch D, Salat C, et al. Treatment of advanced gastrointestinal cancer with genetically modified autologous mesenchymal stem cells: results from the phase 1/2 TREAT-ME-1 trial. Int J Cancer. 2019;145(6):1538–46.
[17] Moll G, Drzeniek N, Kamhieh-Milz J, Geissler S, Volk HD, Reinke P. MSC therapies for COVID-19: importance of patient coagulopathy, thromboprophylaxis, cell product quality and mode of delivery for treatment safety and efficacy. Front Immunol. 2020;11:1091.
[18] Conrad C, Niess H, Huss R, Huber S, von Luettichau I, Nelson PJ, et al. Multipotent mesenchymal stem cells acquire a lymph endothelial phenotype and enhance lymphatic regeneration in vivo. Circulation. 2009;119(2):281–9.
[19] Ankrum JA, Ong JF, Karp JM. Mesenchymal stem cells: immune evasive, not immune privileged. Nat Biotechnol. 2014;32(3):252–60.
[20] Sarkar D, Spencer JA, Phillips JA, Zhao W, Schafer S, Spelke DP, et al. Engineered cell homing. Blood. 2011; 118(25): e184-191.

Copyright - Unless otherwise stated all contents of this website are AcceGen™ All Rights Reserved – Full details of the use of materials on this site please refer to AcceGen Editorial Policy – Guest Posts are welcome, by submitting a guest post to AcceGen you are agree to the AcceGen Guest Post Agreement – Any concerns please contact [email protected]