- In-Stock Tumor Cell Lines
- Human Orbital Fibroblasts
- Human Microglia
- Human Pulmonary Alveolar Epithelial Cells
- Human Colonic Fibroblasts
- Human Type II Alveolar Epithelial Cells
- Human Valvular Interstitial Cells
- Human Thyroid Epithelial Cells
- C57BL/6 Mouse Dermal Fibroblasts
- Human Alveolar Macrophages
- Human Dermal Fibroblasts, Adult
- Human Lung Fibroblasts, Adult
- Human Retinal Muller Cells
- Human Articular Chondrocytes
- Human Retinal Pigment Epithelial Cells
- Human Pancreatic Islets of Langerhans Cells
- Human Kidney Podocyte Cells
- Human Renal Proximal Tubule Cells
Oncology field has been revolutionized by the emergence of cellular immunotherapies that augment the natural capacity of the immune system for against cancer. However, for tumor-bearing patients, this ability is often impaired. Adoptive T-cell therapy aims to bolster deficient endogenous immune responses directly, providing a promising scientific research direction.
For example, CAR-expressing T-cells (CAR-Ts) is such an adoptive T-cell therapy designed by joining chimeric antigen receptors (CARs) with immune responsive T-cell. CARs are composed of antigen recognition domains, which are derived from tumor-associated antibodies. With the efforts in gene engineering, CARs are joined to an internal T-cell signaling domain, helping T cell recognize its antigen targets through a mechanism different from classical T-cell receptors (TCRs). CAR-T technology has allowed the introduction of a high degree tumor selectivity into adoptive cell transfer therapies, combined the strengths of cellular and humoral immunity, and equipped the patient’s immune cell with unique and strengthened tumor-specific recognition chains. These properties enhanced the functions of T cells, such as obtaining superior cytotoxicity, persistence, and antigen recognition capabilities in the face of tumor-induced immunosuppressive influences.


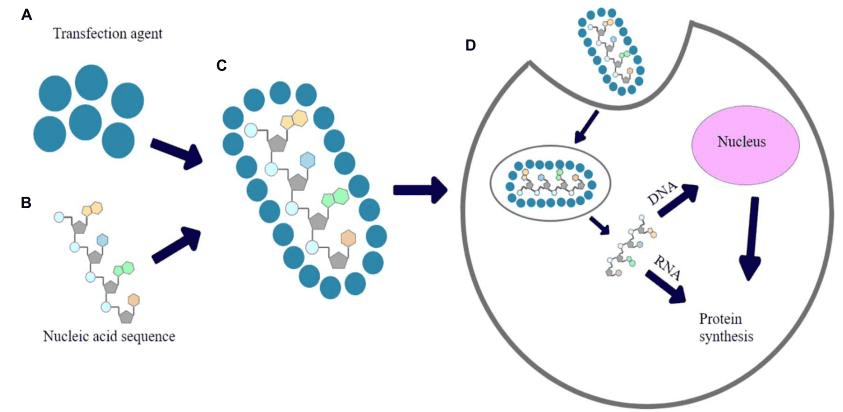
Fig 1. CAR-T therapy introduction
(a) The structure of CAR-T cells (b) The main process of CAR-T therapy
As one of the most promising cancer immunotherapy modalities, CAR-Ts has demonstrated a remarkable antitumor efficacy, especially in the treatment of hematologic cancers. CD19 is a B-cell surface expression antigen, it has also been regarded as a viable target of CAR-T therapy. CD19 and CD20 targeting CAR-T therapy have been successfully used in the treatment of hematologic malignancies, by sustaining antitumor immune responses and producing sustained tumor regressions in the patients with acute lymphoblastic leukemia (ALL), chronic lymphocytic leukemia (CLL), multiple myeloma, or treatment-refractory diffuse large B-cell lymphoma (DLBCL).
Inspired by the success in liquid tumors therapy, researchers have a great interest in expanding the use of CAR-T technology to treat solid tumors.
The Current Difficulties in CAR-T for solid tumor
However, the identification of commonly expressed antigen targets remains a constant challenge. Across different patients, the high degree of solid tumor heterogeneity exists, which makes it difficult to find such an antigen broadly applicable for CAR-T immunotherapy. Barrier to the efficacy of CAR-T in solid tumors is a characteristic state of profound tumor-induced suppression of host antitumor immunity.

Fig 2. The mechanism of PD-1 targeting CAR-T therapy
PD-1 targeting CAR-T therapy
One potential solution is to study the individual intertumoral mutation profiles, which has revealed an exciting new category of possible antigen targets that may allow for the development of highly specific and effective personalized therapies Recently, the surface expression of immunosuppressive molecules attracts the scientists’ attention. These molecules inhibit functions of T-cell effector directly by exploiting endogenous regulatory pathways. Among these immune checkpoints, cytotoxic T-lymphocyte antigen-4 (CTLA-4) pathways and the programmed cell death-1 (PD-1) become research hotspots. Activation of CTLA-4 receptors expressed by naive T cells prevents the initial activation and stimulation of PD-1 on activated T-cells which induce apoptosis or development of immunosuppressive regulatory T-cells (Tregs). By upregulating PD-L1 and enhancing T-cell CTLA-4 and PD-1 expression, tumor cells are able to suppress the activity of incoming immune cells. The studies show a common mechanism of immune escape provides a possible target of eliminating solid tumor.
Multiple targeting CAR-T therapy
Multiple targeting CAR-T also has the potential to enhance the specificity for tumor cells, lessen the risk of off-target effects, and reduce the emergence of antigen loss variants and therapy-resistant tumors. After studying single cell co-expression patterns of the HER2, IL-13Rα2 and EphA2 in primary glioblastoma (GBM) samples, Hegde et al. developed a mathematical model of antigen expression to predict the odds of tumor elimination with CAR-T therapy. These CAR-T cells co-targeting HER2 and IL-13Rα2, without a third antigen, exhibited enhanced antitumor activity and resistance to antigen escaping in in vitro immunoassays and an orthotopic xenogeneic murine model. Due to the risk of damaging surrounding eloquent brain tissue, GBM is considered as an intractable disease cause it’s dangerous to remove lesion with relatively nonspecific therapies like surgery and radiation. The CAR-T therapy can be a breakthrough by targeting the multiple antigen with higher precision.
This technique may be particularly effective against tumors that overexpress two antigens, these antigens are individually not exclusively expressed by malignant cells but result in acceptable tumor specificity cells when coupled together.
Tumor cell selectivity of bi-specific CAR T-cells can be further refined to only targeting cells expressing a specific antigen combination. This is achieved by physically separating CAR signaling domains between two distinct CARs specific for two different antigens, and binding of both CARs to their individual target antigens is required to transmit an activating signal to induce T-cell cytotoxicity.
Although target selection still plaguing CAR-T in the treatment of solid tumors, CAR-T therapy has significant potential to push the progress of treating solid tumors forward. Strategies that aim at the unique aspects of solid tumor antigen like molecular heterogeneity, isolated tumor common feature, bi-specific etc. could be further studied to extend the use of CAR-T immunotherapy to solid tumors.
AcceGen CAR-T cell Custom Service
Immunotherapy like CAR-T has become a revolution in the area of cancer treatment. AcceGen is on target to be a reliable provider for immunotherapy, to relieve the burden of cancer treatment and to improve human health.
Currently, AcceGen offers the CAR-T cell custom service that targeting various biomarkers to fit your requirements. We continue to focus on the field of immunotherapy, especially CAR-T and to update our products.
If you have any questions, please call us at 1-862-686-2696 or send an email to [email protected]. Your questions and requests will be answered by expert staff in AcceGen within 24 hours.

Copyright - Unless otherwise stated all contents of this website are AcceGen™ All Rights Reserved – Full details of the use of materials on this site please refer to AcceGen Editorial Policy – Guest Posts are welcome, by submitting a guest post to AcceGen you are agree to the AcceGen Guest Post Agreement – Any concerns please contact [email protected]