- In-Stock Tumor Cell Lines
- Human Orbital Fibroblasts
- Human Microglia
- Human Pulmonary Alveolar Epithelial Cells
- Human Colonic Fibroblasts
- Human Type II Alveolar Epithelial Cells
- Human Valvular Interstitial Cells
- Human Thyroid Epithelial Cells
- C57BL/6 Mouse Dermal Fibroblasts
- Human Alveolar Macrophages
- Human Dermal Fibroblasts, Adult
- Human Lung Fibroblasts, Adult
- Human Retinal Muller Cells
- Human Articular Chondrocytes
- Human Retinal Pigment Epithelial Cells
- Human Pancreatic Islets of Langerhans Cells
- Human Kidney Podocyte Cells
- Human Renal Proximal Tubule Cells
With the advancement of cell culture technology, the application of primary cells isolation and culture has gradually become common after the 2000s. Compared with the immortalized cell line, which can be cultured in vitro for a long time, the primary cells are closer to the actual physiological process in vivo, especially in the project based on mouse models. Therefore, mouse primary cells became a more general choice for researchers in project design based on mouse models.
What are Primary Cells?
Primary cells are freshly obtained from a multicellular organism and cultured in-vitro cells. Unlike immortalized cell lines, primary cells cannot be subcultured indefinitely due to senescent-phenotype-induced irreversible cell cycle arrest. The survival and growth of primary cells have higher requirements for culture substrates and nutrient conditions so that their applications are not popularized until the 21st century.
Primary cells present several advantages over immortalized cell lines, including a better representation of the cellular heterogeneity of samples, a more faithful transcriptomic and proteomic profile and more realistic functional responses. Therefore, primary cells are a valuable tool for physiology or pathology and clinical research [1].
Primary Cells in Mouse Models
Mouse model is one of the most common research objects in biomedical research. Its advantages include low cost, quick reproduction and high genetic similarity to humans [2]. In vivo experiments based on mouse models and in vitro experiments on mouse primary cells are becoming more widely used.
Primary mouse cells are the ideal research object for in vitro experiments corresponding to in vivo experiments. Many physiological functions can be reproduced in vitro more accurately by mouse primary cells. Besides, the isolation and culture of primary mouse cells are necessary steps for in vivo experimental cell-level analysis of mouse models, such as flow cytometry (FCM) [3].
Isolation of Mouse Primary Cells and In Vitro Culture
Generally, the basic technical process of the mouse primary cells isolation includes digestion, extraction, purification, viability assessment, quantitation and overnight culture, as shown in Figure1(a). And it is necessary to pay attention to avoiding the contamination of bacteria during the operation.
Here we take the isolation of mouse primary hepatocytes as an example. Sequentially pump Hanks’ Balanced Salt Solution (HBSS) and digestion medium into the liver through the portal vein to digest the tissue, and the liver will be blanched and swell. Tear apart the lobes of the liver to free the cells into the digestion medium and then purify the medium to remove the residue through filtration and washing. Stain the obtained mouse primary hepatocytes with 0.4% trypan blue for viability identification and count. Then plate the cells and culture overnight. This brief operation process is shown in Figure 1(b), and the detailed protocol can be checked in http://mouselivercells.com/procedure.html [3].
The overall operating principle of primary cells isolation for different tissues is roughly the same, but the detailed steps are different. For example, during the isolation of mouse primary enterocytes cells, the intestinal tissues need to be washed repeatedly with clean HBSS buffer to prevent contamination from the gut microbes [4].
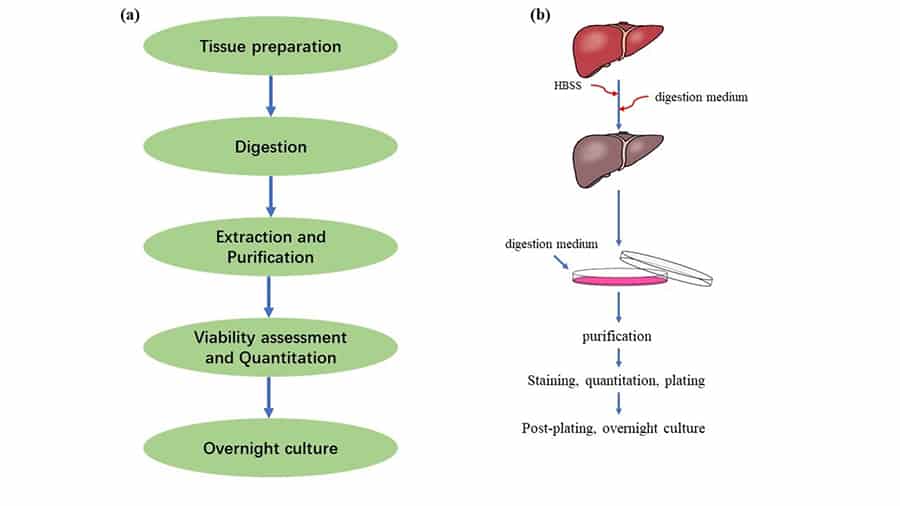
Figure 1. Mouse primary cell extraction and culture. (a) The technical process of mouse primary cell extraction and culture. (b) Isolation and culture process of primary mouse Hepatocytes[3].
Application of Mouse Primary Cells
Mouse models and primary cells based on different strains apply to different research fields. C57BL/6 mouse strain is the most widely used strain due to its stability and easy breeding. C57BL/10J (the substrain of C57BL/6) and B6129SF2/J (hybrid strain between mouse C57BL/6 and B129/S) are derivative strains of C57BL/6As an easy breeding and immunodeficient strain, Balb/c is another strain used widely in hybridoma and monoclonal antibody production and immunology research. Apart from inbred strains, outbred stock CD1 is another commonly used strain that applies to positional cloning and genotypic selection. The information of these strains/stock is shown in Table 1[2].
Table 1. Mouse strains and characteristics
| Strain/stock | Features | Applications | Reference |
| C57BL/6 | inbred, black | physiological or pathological models for in vivo experiments, background strain for transgenics and congenics. | [2] |
| BALB/c | inbred, albino, immunodeficient | hybridoma and monoclonal antibody production, research models for cancer therapy and immunology. | [2] |
| CD1 | outbred, albino | positional cloning, genotypic selection. | [2, 5] |
| B6129SF2/J | C57BL/6 × B129/S, hybrid strain | map traits, approximate controls for genetically engineered strains. | [6, 7] |
| C57BL/10J | Inbred, black | immunological research. | [8] |
– The Application of C57BL/6 Primary Cells in the Research of Non-alcoholic Steatohepatitis (NASH)
In this publication, the researchers isolated the primary mouse hepatocytes from C57BL/6J. They transduce inositol-requiring enzyme-1A (IRE1A) into the primary mouse hepatocytes through virus to identify the role of IRE1A in vitro. Combining the results of in vivo experiments in mouse models and in vitro experiments in mouse primary hepatocytes, they found that the activation of IREA1 can induce liver inflammation and injury in diet-induced steatohepatitis mice. The mechanism is the transcription of serine palmitoyl transferase genes promoted by IREA1 through X-box binding protein 1 (XBP1) leads to ceramide biosynthesis and extracellular vesicles (EVs) released. And then, EVs can induce liver inflammation and injury by recruiting monocyte-derived macrophages to the liver [9]. (Figure 2)
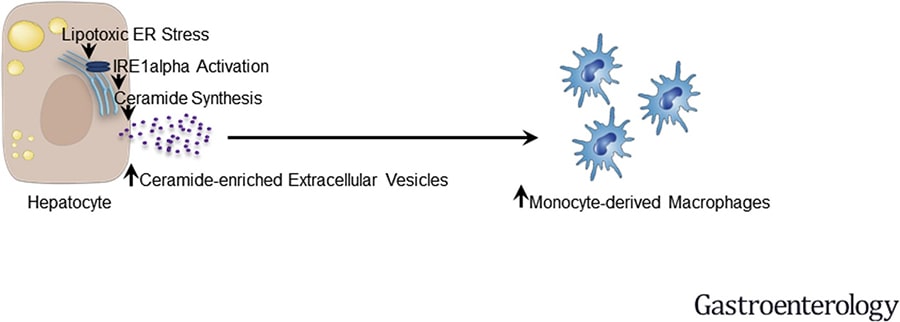
Figure 2. The application of C57BL/6 mouse primary hepatocytes in the research of NASH[9].
– The Application of BALB/c Primary Cells in the Research of Intestinal Immune Response
Primary mouse small intestine epithelial cells were isolated from BALB/c mouse and cultured in vitro. The cells were treated with Active Hexose Correlated Compound (AHCC) and anti-TLR-2 and TLR-4 blocking antibodies to examine the effect and the mechanism of AHCC in vitro. LPS or E. coli were used to induce the immune reaction of the cells. Combining in vivo experiments of BALB/C mice fed AHCC, they found that AHCC can orchestrate immune response and maintain immune homeostasis. And the potential mechanism of this process is TLR-2 and TLR-4 pathway [4].
Conclusion
Mouse primary cells are a valuable tool in the research of physiology and pathology as well as the clinical research. Although it is complicated and difficult to isolate and culture in vitro, mice cells have unique advantages of providing more reliable in vitro experimental data in the works based on mouse models.
Where to Get Mouse Primary Cells for research?
Mouse primary cells play a critical role in in vitro experiments. AcceGen provides the most authentic Mouse Primary Cells to meet all your research needs. We have rich mouse cell models, such as C57BL/6, Balb/C, CD1 and so on. It is our pleasure to help relative research to move forward. All the products of AcceGen are strictly comply with international standards. For more detailed information, please visit our product portfolio or contact [email protected].
Reference
1. Wikipedia: https://en.wikipedia.org/wiki/Primary_cell_culture.
2. Johnson M: Laboratory Mice and Rats. MATER METHODS 2012, 2:113.
3. Zhang W: Primary Mouse Hepatocytes. http://mouselivercellscom/abouthtml.
4. Mallet JF, Graham É, Ritz BW, Homma K, Matar C: Active Hexose Correlated Compound (AHCC) promotes an intestinal immune response in BALB/c mice and in primary intestinal epithelial cell culture involving toll-like receptors TLR-2 and TLR-4. Eur J Nutr 2016, 55:139-146.
5. J:ARC(S).
https://wwwjaxorg/strain/034608?utm_source=google&utm_medium=ppc&utm_campaign=swiss-outbred&gclid=Cj0KCQjwvO2IBhCzARIsALw3ASoE53iwFrshMt-JwgbVUUbgR_ELm4skTUYX6_irYJQQb93pJTF74XAaAsaTEALw_wcB.
6. Simpson EM, Linder CC, Sargent EE, Davisson MT, Mobraaten LE, Sharp JJ: Genetic variation among 129 substrains and its importance for targeted mutagenesis in mice. Nat Genet 1997, 16:19-27.
7. B6129SF2/J: https://www.jax.org/strain/000665. Jackson Lab.
8. C57BL/10J. https://wwwjaxorg/strain/000665.
9. Dasgupta D, Nakao Y, Mauer AS, Thompson JM, Sehrawat TS, Liao CY, Krishnan A, Lucien F, Guo Q, Liu M, et al: IRE1A Stimulates Hepatocyte-Derived Extracellular Vesicles That Promote Inflammation in Mice With Steatohepatitis. Gastroenterology 2020, 159:1487-1503.e1417.

Copyright - Unless otherwise stated all contents of this website are AcceGen™ All Rights Reserved – Full details of the use of materials on this site please refer to AcceGen Editorial Policy – Guest Posts are welcome, by submitting a guest post to AcceGen you are agree to the AcceGen Guest Post Agreement – Any concerns please contact [email protected]