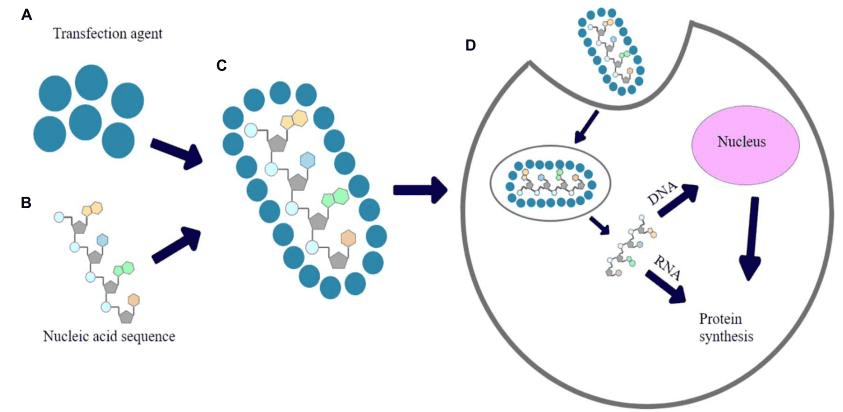
- In-Stock Tumor Cell Lines
- Human Orbital Fibroblasts
- Human Microglia
- Human Pulmonary Alveolar Epithelial Cells
- Human Colonic Fibroblasts
- Human Type II Alveolar Epithelial Cells
- Human Valvular Interstitial Cells
- Human Thyroid Epithelial Cells
- C57BL/6 Mouse Dermal Fibroblasts
- Human Alveolar Macrophages
- Human Dermal Fibroblasts, Adult
- Human Lung Fibroblasts, Adult
- Human Retinal Muller Cells
- Human Articular Chondrocytes
- Human Retinal Pigment Epithelial Cells
- Human Pancreatic Islets of Langerhans Cells
- Human Kidney Podocyte Cells
- Human Renal Proximal Tubule Cells
Proximal tubule (PT) and PT Cells
The proximal tubule (PT) is a part of the renal tubule and a segment of the nephron (Figure.1) in kidneys, which consists of two parts: the convoluted tubule and the straight tubule[1]. The residual nutrients from filtrate are reabsorbed by PT, including nearly 80% of glucose, more than 50% of salt, and all filtered organic solutes (amino acids)[2]. PT can regulate the pH of the filtrate by secreting hydrogen ions (acid) into the tubule and reabsorbing nearly 80% of the bicarbonate[3]. The structure of PT’s inner epithelium shows densely fluffy, which forms a large superficial area to enhance the functions of reabsorptive and putative flow sensing[4]. These structure and functional characteristics also determine the composition of the cells within the PT epithelium.
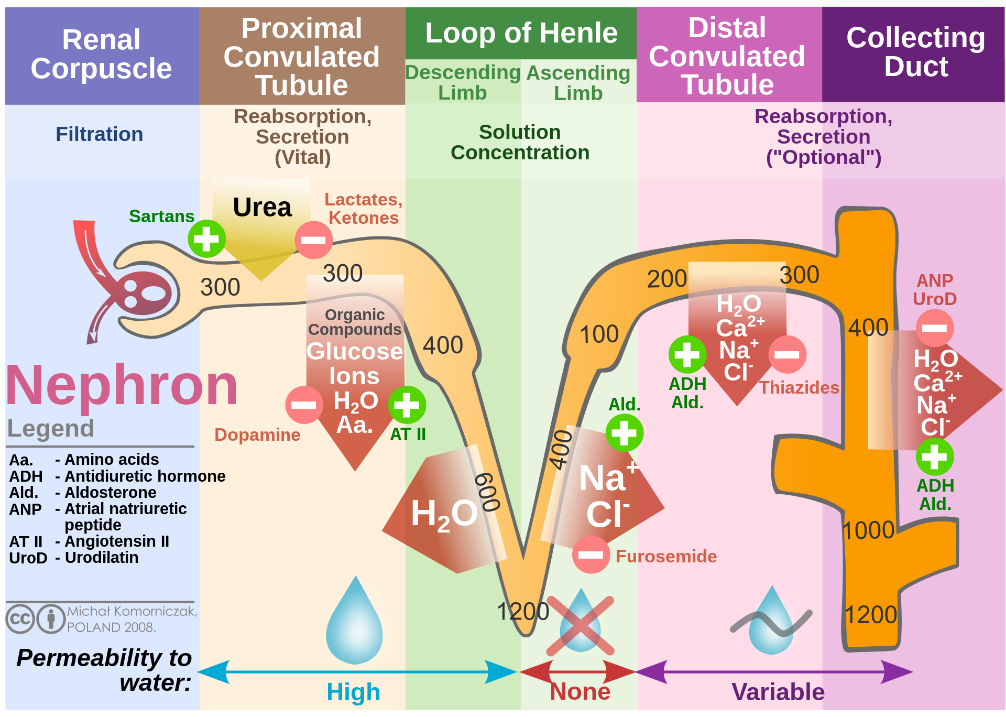
Figure.1 Renal Nephron
Renal proximal tubule cells constitute the function and structure of the PT. Besides the microvillus structure on the surface, enrichment of a large number of mitochondria in the cytoplasm is also a characteristic of PT cells. Enriched mitochondria provide sufficient energy for the active transport of sodium ions across membranes, which establishes a salt concentration gradient to support the reabsorption of salt. Therefore, mitochondrial dysfunction is closely related to kidney pathological processes[5]. Renal fibrosis is one of the main features of PT-related chronic kidney diseases, which are frequently associated with metabolic syndrome (mainly diabetes) and exposure to toxins or excessive drugs[6; 7]. The renal tubule, especially PT cells, is considered to be the beginning of renal fibrosis, which shows the importance of PT cells in kidney pathology[8].
Human Renal Proximal Tubule Cells and Culture Protocol
Renal proximal tubule (epithelium) cells (PTECs) in vitro models play key roles in the research of kidney physiology, disease pathology, and pharmacology[9]. Immortalized cell lines are the widely used in vitro models. These cell lines are straightforward to cultivate and apply, but immortality transformation can inevitably disrupt some physiological functions of cells, such as the expression deletion of organic anion transporters. Primary PTECs, especially human source primary cells are one of the reliable in vitro models, although these cells are not readily available and difficult to cultivate in vitro. Furthermore, pluripotent stem cell-derived PTECs are emerging in vitro models in recent years, which provide another choice for researchers. In vitro, 3D cultures, or organoids based on PTECs are the next generation of in vitro models, which can in vitro simulate the physiological structure of PT in vivo.
In these in vitro models based on PTECs, human primary PTECs are a reliable choice. Therefore, we give a brief introduction to the isolation and culture protocol of human primary PTECs in this section[9]. The fresh tissue was firstly micro-dissection (mechanical pre-processing) and then digested by collagenase type I or hyaluronidase. After the sieve filtration, the PTECs was isolated preliminary from various kidney cell types by density-gradient centrifugation, and cultured in the medium (DMEM/F12 with FBS, penicillin/streptomycin, human transferrin, selenium, hydrocortisone, insulin, and epidermal growth factor). PTECs were more than 95% of proximal tubular origin in this culture and further purification was achieved through trypsinization and flowcytometric sorting for the proximal tubular marker leucine aminopeptidase (LAP). Primary PTECs require approximately 10-13 days to reach confluence after seeding, while subculture cells have a doubling time of 24-48 hours and reach confluence in 3-5 days.
Application of Human PTECs in the publications
Diabetic nephropathy (DN) is a prevalent PT-relate kidney diseases, caused by diabetes-induced albuminuria, leading to kidney injury. The study conducted by Zeng, et al. revealed that ORAI channels of PTECs are critical for the endocytosis of albumin [10]. The researchers used HK-2 cell lines and primary human PTECs as in vitro models, while Akita type 1 diabetic mice (C57BL/6-Ins2Akita/J) and proximal tubule-specific expression of ORAI1 dominant-negative mutant mice (DN-Orai1E108Q) were used as in vivo models. Firstly, the study revealed that ORAI1-3 was preferentially expressed but downregulated in DN patients, and the expression of ORAI1-3 was reduced by hyperglycemia or blockade of insulin signaling. Then, the research demonstrated that the inhibition of ORAI expression and blocking of the ORAI channel impaired albumin uptake in human primary PTECs. In mouse models, both C57BL/6-Ins2Akita/J blocking ORAI channel and DN-Orai1E108Q showed exacerbated albuminuria. Furthermore, the potential molecular mechanisms of ORAI-related exacerbation of albuminuria. The albumin reabsorption of PTECs relied on receptors-clathrin-induced endocytosis, which depended on the ORAI-AMN-STIM1 complex and Ca2+ transmembrane flow. DN-induced excessive albumin possibly decreased the activity of store-operated Ca2+ entry in the cells and then weakened ORAI-dependent albumin endocytosis, ending up with exacerbating albuminuria. In a previous publication, Tang, et al. found that albumin induced inflammation reaction of PTECs[11]. The researchers used human primary PTECs from kidneys removed for circumscribed tumors as in vitro models and identified that albumin stimulated interleukin-8 expression in PTECs. These publications showed the crucial roles of PTECs in kidney and global metabolic diseases, and revealed the significant importance of human primary PTECs in research.
Conclusion
The kidney is an important organs in global metabolism. Kidney diseases and injury are frequently accompanied by metabolic syndrome act as triggers for metabolic disorder, and PT plays an essential role in these processes. Therefore, PTECs are an essential research object in the research of kidney, which means that it is significant for researchers engaged in kidney disease research to master the relevant application methods and techniques of PTECs in vitro models.
Where to Get Urinary System Primary Cells for Your Research?
AcceGen isolates and offers a wide range of high-quality urinary system primary cells, such as Human Renal Proximal Tubule Cells, Human Renal Fibroblasts, Human Renal Tubular Epithelial Cells, and Human Renal Glomerular Endothelial Cells. These cell products provide you with a convenient means to research. To get more information, please refer to: Urinary System Primary Cells.
It is our pleasure to help relative researches to move forward. All the products of AcceGen are strictly comply with international standards. For more detailed information, please visit our product portfolio or contact [email protected].
References
[1] M.H. Ross, and W. Pawlina, Histology: A Text and Atlas: with Correlated Cell and Molecular Biology, Lippincott Williams & Wilkins, 2015.
[2] A. Mescher, JUNQUEIRAS BASIC HISTOLOGY 14E, McGraw Hill LLC, 2015.
[3] W.F. Boron, Acid-Base Transport by the Renal Proximal Tubule. Journal of the American Society of Nephrology 17 (2006) 2368-2382.
[4] T. Wang, Flow-activated transport events along the nephron. Curr Opin Nephrol Hypertens 15 (2006) 530-6.
[5] T. Doke, and K. Susztak, The multifaceted role of kidney tubule mitochondrial dysfunction in kidney disease development. Trends Cell Biol 32 (2022) 841-853.
[6] Y.S. Han, Y.M. Yoon, G. Go, J.H. Lee, and S.H. Lee, Melatonin Protects Human Renal Proximal Tubule Epithelial Cells Against High Glucose-Mediated Fibrosis via the Cellular Prion Protein-TGF-β-Smad Signaling Axis. Int J Med Sci 17 (2020) 1235-1245.
[7] Y.M. Yoon, G. Go, C.W. Yun, J.H. Lim, and S.H. Lee, Knockdown of CK2α reduces P-cresol-induced fibrosis in human renal proximal tubule epithelial cells via the downregulation of profilin-1. Int J Med Sci 17 (2020) 2850-2860.
[8] B.C. Liu, T.T. Tang, L.L. Lv, and H.Y. Lan, Renal tubule injury: a driving force toward chronic kidney disease. Kidney Int 93 (2018) 568-579.
[9] M. Mihevc, T. Petreski, U. Maver, and S. Bevc, Renal proximal tubular epithelial cells: review of isolation, characterization, and culturing techniques. Molecular Biology Reports 47 (2020) 9865-9882.
[10] B. Zeng, G.L. Chen, E. Garcia-Vaz, S. Bhandari, N. Daskoulidou, L.M. Berglund, H. Jiang, T. Hallett, L.P. Zhou, L. Huang, Z.H. Xu, V. Nair, R.G. Nelson, W. Ju, M. Kretzler, S.L. Atkin, M.F. Gomez, and S.Z. Xu, ORAI channels are critical for receptor-mediated endocytosis of albumin. Nat Commun 8 (2017) 1920.
[11] S. Tang, J.C. Leung, K. Abe, K.W. Chan, L.Y. Chan, T.M. Chan, and K.N. Lai, Albumin stimulates interleukin-8 expression in proximal tubular epithelial cells in vitro and in vivo. J Clin Invest 111 (2003) 515-27.

Copyright - Unless otherwise stated all contents of this website are AcceGen™ All Rights Reserved – Full details of the use of materials on this site please refer to AcceGen Editorial Policy – Guest Posts are welcome, by submitting a guest post to AcceGen you are agree to the AcceGen Guest Post Agreement – Any concerns please contact [email protected]