- In-Stock Tumor Cell Lines
- Human Orbital Fibroblasts
- Human Microglia
- Human Pulmonary Alveolar Epithelial Cells
- Human Colonic Fibroblasts
- Human Type II Alveolar Epithelial Cells
- Human Valvular Interstitial Cells
- Human Thyroid Epithelial Cells
- C57BL/6 Mouse Dermal Fibroblasts
- Human Alveolar Macrophages
- Human Dermal Fibroblasts, Adult
- Human Lung Fibroblasts, Adult
- Human Retinal Muller Cells
- Human Articular Chondrocytes
- Human Retinal Pigment Epithelial Cells
- Human Pancreatic Islets of Langerhans Cells
- Human Kidney Podocyte Cells
- Human Renal Proximal Tubule Cells
Introduction: Esophagus and Diseases
Esophagus is an essential part of the digestive tract and plays an indispensable role in the eating and food digestion and absorption process. Therefore, esophageal diseases can seriously affect the digestive ability and even lead to life-threatening consequences.
Esophagus
Esophagus is located at the upper part of the digestive system, the upper part is connected to the pharynx and the lower part to the cardia of the stomach, which is the passage of food and drink into the stomach[1; 2]. (Figure.1) As the first part of the digestive system and gastrointestinal tract, the esophagus transports food after oral chewing from the pharynx to the stomach through the swallowing function[3]. And esophagus can prevent gastric reflux through mechanical shrinkage capacity to maintain the digestive tract homeostasis[4]. The mechanical contraction ability of the esophagus to swallow and prevent gastric reflux is attributed to the two main sphincters at the top and bottom of the esophagus, which are the upper esophageal sphincter and the lower esophageal sphincter[3]. And the contraction of the esophageal sphincter is controlled by the sympathetic nerve and vagus nerve, such as the swallowing reflex[5]. Besides, the gastro-esophageal junction is another key part of the esophagus, the mucosal morphology suddenly transitions from the esophagus to the stomach here, and the dividing line is called the z-line, and the functional area of the lower esophageal sphincter is just below the z-line[5].
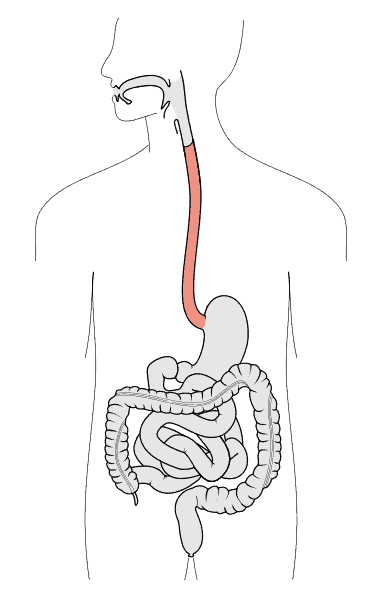
Figure.1 Esophagus[6].
Esophageal diseases
Esophageal diseases mainly start from inflammation, and chronic inflammation is the main cause of esophageal cancer. Factors that can induce the inflammation of the esophagus mainly include gastric acid reflux, exogenous infection, drugs, food allergy, and substance intake[7]. Long-term chronic inflammation of the esophagus induced by gastric reflux may lead to Barrett esophagus, which is thought to be one of the main causes of esophageal cancers. (Figure.2) Gastric reflux makes the lower esophagus long-term exposure to an acidic environment, leading to the z-line move up, which is the typical characterization of Barrett esophagus[8]. These diseases are often precursors to cancer and are prone to develop into cancer without proper clinical treatment and attention. Esophageal cancer mainly includes squamous cell carcinoma or adenocarcinoma. Squamous cell carcinoma is closely related to cigarette and alcohol, and adenocarcinoma mainly relates to gastric reflux[9]. Besides, esophageal varices, esophageal achalasia, and esophageal deformation are also diseases that affect esophageal function[1; 7; 10].
(a)

(b)

Figure.2 (a) Gastroscopic imaging of Barrett esophagus. (b) Pathological staining of Barrett esophagus.
Human Esophageal Epithelial Cells and Culture Methods
Both esophageal primary cells and immortalized cell lines are valuable tools as in vitro models for studying esophagus-related diseases and physiological mechanisms. Immortalized cell lines are mainly established by primary cells transfected with SV40 large T-antigen, and tumor cell lines are mainly isolated from clinical biopsy samples. Although immortalized cell lines are wider application due to easy cultivation, the genetic background will be altered during the process of immortalization transformation and long-term transmission, thereby affecting the reliability of experimental results. For example, Wang et al. found that sterigmatocystin induces G1 arrest in primary human esophageal epithelial cells but induces G2 arrest in immortalized cell lines[11]. Therefore, primary esophageal cells remain one of the most reliable in vitro models for research.
Here, we will give a brief introduction to the isolation and culture protocol of human primary esophageal epithelial cells[11]. The cells are isolated from normal esophageal tissue harvested from an esophagectomy specimen. The specimen needs to be transported in Hank’s Balanced Salt Solution with penicillin and streptomycin, and washed several times with PBS, and placed in PBS with amphotericin B before the next step. After incubation with dispase at 4℃ overnight, the epithelium was dissected and then digested with with trypsin for 30min. After termination of digestion and centrifugation, resuspend the cells with culture medium and culture at 37℃ with 5% CO2 after plating. The composition of the culture medium is keratinocyte-SFM medium with bovine pituitary extract, EGF, and penicillin&streptomycin. Primary cells are usually slow growing and have a limited number of passages (less than ten generations), and esophageal cancer cell lines (such as KYSE510, TE-1, ECA109, etc.) are also valuable in vitro models in the research of pathology of esophageal cancer.
Application of Human Esophageal Epithelial Cells in Publication
Eosinophilic esophagitis (EoE) is an esophageal allergic disease that manifests as a dysfunction of the esophageal epithelium caused by an epithelial immune response induced by food allergens. Ruffner et al. found that the Toll-like receptor 2 (TLR2) pathway is a potential regulation factor of esophageal epithelial barrier function in EoE[12]. They isolated the human primary esophageal epithelial cells from EoE patients and analyzed the expression of pattern recognition receptors, and found high expression levels of TLR2 and TLR3. Then, they estimate the epithelial barrier function under TLR2 and TLR3 stimulation through three‐dimensional air‐liquid interface culture (ALI) model of human epithelium in vitro model. They found that TLR2 but not TLR3 upregulates the transepithelial electrical resistance (TEER) and decreased paracellular permeability to FITC-Dextran, and this phenomenon can be eliminated by TLR2 blocking. Furthermore, they found the upregulation of TJ complex proteins claudin-1 and zonula occludens-1 following TLR2 stimulation, and ChIP analysis shows persistent chromatin changes in this process. All in all, these results indicated that the TLR2 pathway is a potential regulator of the esophageal epithelial barrier in EoE, and this process is related to the chromatin modification of TLR2 downstream.
Conclusion
Esophagus is the first part of the digestive system, and esophagus diseases seriously affect daily life, sometimes leading to serious consequences, which makes esophagus diseases a hot research field. As powerful and reliable in vitro models, human esophageal epithelial cells can provide reliable data for researchers in the research of esophagus diseases, which means it is necessary for the researchers to master the usage methods.
Where to Get Digestive System Primary Cells for Your Research?
AcceGen isolates and offers a wide range of high-quality digestive system primary cells, such as Human Esophageal Epithelial Cells, Human Oral Epithelial Cells, Human Kupffer Cells, and Human Gastric Epithelial Cells. These cell products provide you with a convenient means to research. To get more information, please refer to: Digestive System Primary Cells.
It is our pleasure to help relative researches to move forward. All the products of AcceGen are strictly comply with international standards. For more detailed information, please visit our product portfolio or contact [email protected].
References
[1] D. Purves, Neuroscience, Oxford University Press, 2012.
[2] R.L. Drake, W. Vogl, and A.W.M. Mitchell, Gray’s Anatomy for Students, Elsevier/Churchill Livingstone, 2005.
[3] A.C. Guyton, and J.E. Hall, Textbook of Medical Physiology, Elsevier Saunders, 2006.
[4] E. Mittal RK. Motor Function of the Pharynx, and its Sphincters. San Rafael (CA): Morgan & Claypool Life Sciences; 2011. Neuromuscular Anatomy of Esophagus and Lower Esophageal Sphincter. Available from: https://www.ncbi.nlm.nih.gov/books/NBK54272/.
[5] L. Mu, J. Wang, H. Su, and I. Sanders, Adult human upper esophageal sphincter contains specialized muscle fibers expressing unusual myosin heavy chain isoforms. J Histochem Cytochem 55 (2007) 199-207.
[6] https://en.wikipedia.org/wiki/File:Tractus_intestinalis_esophagus.svg.
[7] N.R. Colledge, B.R. Walker, S. Ralston, and S. Davidson, Davidson’s Principles and Practice of Medicine, Churchill Livingstone/Elsevier, 2010.
[8] N.J. Shaheen, and J.E. Richter, Barrett’s oesophagus. Lancet 373 (2009) 850-61.
[9] P. Lao-Sirieix, C. Caldas, and R.C. Fitzgerald, Genetic predisposition to gastro-oesophageal cancer. Curr Opin Genet Dev 20 (2010) 210-7.
[10] W.J. Larsen, L.S. Sherman, S.S. Potter, and W.J. Scott, Human Embryology, Churchill Livingstone, 2001.
[11] J. Wang, S. Huang, L. Xing, J. Cui, Z. Tian, H. Shen, X. Jiang, X. Yan, J. Wang, and X. Zhang, Sterigmatocystin induces G1 arrest in primary human esophageal epithelial cells but induces G2 arrest in immortalized cells: key mechanistic differences in these two models. Arch Toxicol 89 (2015) 2015-25.
[12] M.A. Ruffner, L. Song, K. Maurer, L. Shi, M.C. Carroll, J.X. Wang, A.B. Muir, J.M. Spergel, and K.E. Sullivan, Toll-like receptor 2 stimulation augments esophageal barrier integrity. Allergy 74 (2019) 2449-2460.

Copyright - Unless otherwise stated all contents of this website are AcceGen™ All Rights Reserved – Full details of the use of materials on this site please refer to AcceGen Editorial Policy – Guest Posts are welcome, by submitting a guest post to AcceGen you are agree to the AcceGen Guest Post Agreement – Any concerns please contact [email protected]