- In-Stock Tumor Cell Lines
- Human Orbital Fibroblasts
- Human Microglia
- Human Pulmonary Alveolar Epithelial Cells
- Human Colonic Fibroblasts
- Human Type II Alveolar Epithelial Cells
- Human Valvular Interstitial Cells
- Human Thyroid Epithelial Cells
- C57BL/6 Mouse Dermal Fibroblasts
- Human Alveolar Macrophages
- Human Dermal Fibroblasts, Adult
- Human Lung Fibroblasts, Adult
- Human Retinal Muller Cells
- Human Articular Chondrocytes
- Human Retinal Pigment Epithelial Cells
- Human Pancreatic Islets of Langerhans Cells
- Human Kidney Podocyte Cells
- Human Renal Proximal Tubule Cells
Hematopoietic stem cells
Hematopoietic stem cells (HSCs), mostly sustained by CD34+CD38– HSCs, are pluripotent suspension stem cells responsible for differentiating into the myeloid and lymphoid progenitor cells that subsequently give rise to all types of blood cells in the body as shown in Figure 1 [1]. These cells have long and short term regenerative properties and can be found in bone marrow. HSCs have powerful clinical relevance regarding diseases related to the lymphatic system in immunology, and diseases related to hematology due to their regenerative abilities.
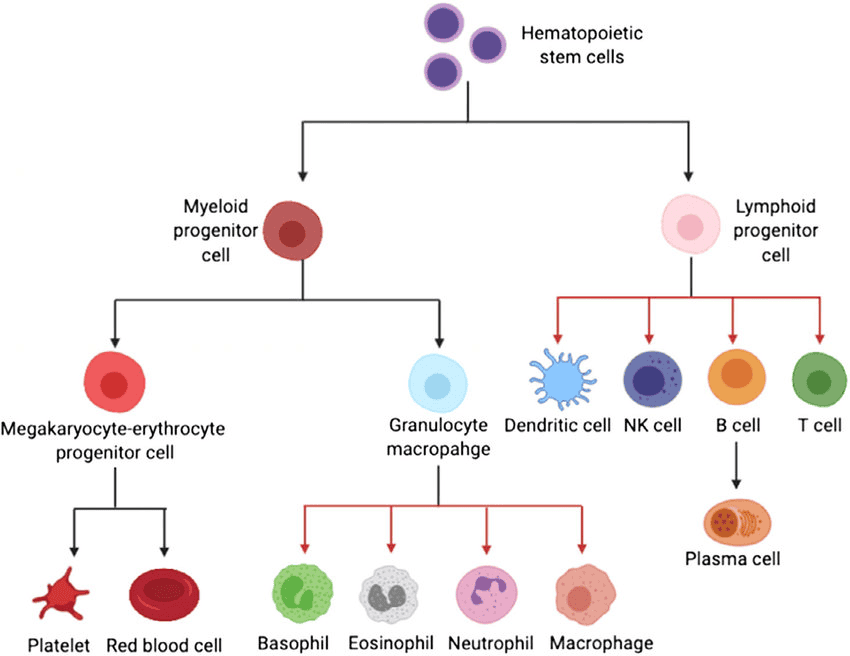
Figure 1. Hematopoietic stem cell differentiation pathways.
HSCs are used in various ways in relation to autologous ex vivo cell and gene therapies. There have been several ongoing clinical trials involving introduction of HSCs for autoimmune diseases, cancers, and rare genetic diseases. Five of the most notable clinical trials being approved by FDA are exagamglogene autotemcel, atidarsagene autotemcel, lovotibeglogene autotemcel, elivaldogene autotemcel, and betibeglogene autotemcel. They are all autologous HSC-based gene therapies aiming at correcting harmful genetic mutations.
Due to the powerful pluripotency of HSCs, isolation and purification of these cells are highly sought after not only from human, but also from other mammals. In this article, methods of isolation and purification of pluripotent HSCs from human will be discussed. Methods and operating procedures critical to research and development of products, as well as the biology behind these procedures will also be addressed.
Isolation and Purification of Human HSCs
Pluripotent stem cells are incredibly valuable to research and development and can be differentiated into any type of cells in the blood for the purpose of regenerative therapeutics [2]. HSCs can be isolated and purified from bone marrow and mobilized peripheral blood which has been treated with mobilizing agents such as granulocyte-colony stimulating factor (G-CSF). HSCs can also be isolated from fetal and embryonic tissues. Although this option is a hotly debated topic of legality, morality and practicality, especially in the United States. In addition, HSCs can be isolated from the cord blood of the umbilical cord and placenta, which are routinely thrown away after the birth of a child.
Among the methods mentioned above, human HSCs isolated and purified from peripheral blood products leukopacks, which are cells in the blood resulting from an enriched apheresis, and peripheral blood mononuclear cells (PBMCs) are more convenient and ethically acceptable. Taking the advantage of flow activated cell sorting (FACS), human HSCs can be easily and efficiently isolated and purified from both leukopacks and PBMCs using different recognizable antigens which are indicative of pluripotent HSCs based on fluorescence-activated cell sorting. So far, human HSCs are most recognized as CD34+, CD38–, cKit–, CD59+, CD90+ and Lin–. Human HSCs are also often referred as CD34+CD38– cells [3].
Culturing and Expansion of Human HSCs
HSCs can be expanded in vitro from very small cell numbers, such as 1,000,000 cells to about 30-40 million cells before they begin to lose their stemness. HSCs are self-renewing cells and retain the ability to provide differentiated progenitors. Numerous types of media and supplements of HSCs have been exploited to maintain their self-renewal, proliferation, and culture under in vitro conditions [3].
The media components needed to expand undifferentiated HSCs consist of DMEM which comprise of four-fold higher concentrations of vitamins, amino acids, and other supplements than Basal Media Eagle’s (BME). The media also has other supplements such as bovine serum albumin, recombinant Human insulin, human transferrin, 2-β-mercaptoethanol, Interlukin-6 (IL6), stem cell colony factor (SCF), Interlukin-3 (IL3), thrombopoietin (TPO) and valproic acid. Serum such as human serum or FBS as well as other growth factors can be included or excluded based on the requirement. Besides DMEM, Iscove’s Modified Dulbecco’s Medium (IMDM) as depicted by Iscove and Guibert, has also been utilized as another significant HSCs culture medium along with various combinations of supplements.
The Use of 3D Hydrogel Matrices for HSC Large-Scale Expansion
Despite the considerable potential of HSCs, large amounts of them are necessary for most studies associated with regenerative applications, drug discovery research, and autologous transplantation. Hydrogel matrices are porous and can be generated from naturally derived macromolecules such as Type 1 collagen, hyaluronic acid, fibrin, and alginate. They can also be made from synthetic polymers polyacrylamide or polyethylene glycol via noncovalent or covalent crosslinking [4].
These 3D hydrogel culturing systems used for stem cells have progressed considerably, and is an improvement of the current 2D stem cell culture methods. Hydrogel matrices have variables that can be manipulated for stiffness, biodegradability and biochemical factors. As shown in Figure 2, such hydrogels can mimic physiological environment and can be considered an artificial bone marrow culture system to obtain large numbers of stem cells used for regenerative therapies [5].
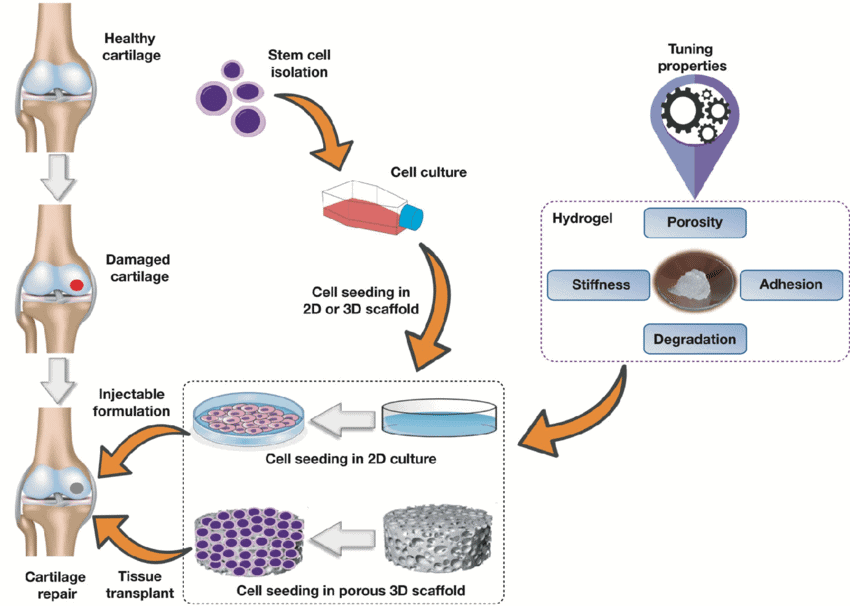
Figure 2. Hydrogel matrix properties and uses.
More research remains to be done to replicate these physiological encapsulations to maintain stemness and optimize differentiation. In addition, drug screening and autologous transplantation of HSCs are also augmented by these hydrogels, since it is difficult to obtain vast numbers of HSCs on 2D-based expansion platforms.
Conclusion
Hematopoietic stem cells is a critical source of therapeutics for regenerative therapies. There are several ways to be considered to isolate and purify human HSCs. Emerging technologies expand these stem cells to a larger scale while maintaining their stemness have augmented HSC clinical applications. These stem cells can also be differentiated into heavily researched cell types necessary for many different types of labs. Isolation, purification and expansion of these stem cells are of great importance and relevance in the field of drug discovery, cell and gene therapies, and regenerative therapies. These cells will continue to be the focus of many personalized therapeutics for the foreseeable future.
| Cat. No | Product Name | Cell Type | |
|---|---|---|---|
| ABC-SC0085T | HighQC™ Human Cord Blood-CD34+ Hematopoietic Stem Cells | Human Hematopoietic Stem Cells | +inquiry |
| ABC-SC0080T | HighQC™ Human CD34+ Hematopoietic Stem Cells (from bone marrow or liver) | Human Hematopoietic Stem Cells | +inquiry |
| ABC-SC0106 | HighQC™ Human Embryonic Hematopoietic Stem Cells | Human Hematopoietic Stem Cells | +inquiry |
References
[1] E.H. Powsner, J.C. Harris, E.S. Day, Biomimetic Nanoparticles for the Treatment of Hematologic Malignancies, Advanced NanoBiomed Research 1(4) (2021).
[2] J.Y. Lee, S.H. Hong, Hematopoietic Stem Cells and Their Roles in Tissue Regeneration, International journal of stem cells 13(1) (2020) 1-12.
[3] P. Yadav, R. Vats, A. Bano, R. Bhardwaj, Hematopoietic Stem Cells Culture, Expansion and Differentiation: An Insight into Variable and Available Media, International journal of stem cells 13(3) (2020) 326-334.
[4] S. Yin, Y. Cao, Hydrogels for Large-Scale Expansion of Stem Cells, Acta biomaterialia 128 (2021) 1-20.
[5] M.M. Rana, H. De la Hoz Siegler, Tuning the Properties of PNIPAm-Based Hydrogel Scaffolds for Cartilage Tissue Engineering, Polymers 13(18) (2021).

Copyright - Unless otherwise stated all contents of this website are AcceGen™ All Rights Reserved – Full details of the use of materials on this site please refer to AcceGen Editorial Policy – Guest Posts are welcome, by submitting a guest post to AcceGen you are agree to the AcceGen Guest Post Agreement – Any concerns please contact [email protected]