- In-Stock Tumor Cell Lines
- Human Orbital Fibroblasts
- Human Microglia
- Human Pulmonary Alveolar Epithelial Cells
- Human Colonic Fibroblasts
- Human Type II Alveolar Epithelial Cells
- Human Valvular Interstitial Cells
- Human Thyroid Epithelial Cells
- C57BL/6 Mouse Dermal Fibroblasts
- Human Alveolar Macrophages
- Human Dermal Fibroblasts, Adult
- Human Lung Fibroblasts, Adult
- Human Retinal Muller Cells
- Human Articular Chondrocytes
- Human Retinal Pigment Epithelial Cells
- Human Pancreatic Islets of Langerhans Cells
- Human Kidney Podocyte Cells
- Human Renal Proximal Tubule Cells
As of 2018, bladder cancer has affected 1.6 million people worldwide, with more than 500,000 new cases and more than 200,000 deaths in recent years. As one of TOP10 malignant and TOP15 cause death cancer [1], bladder cancer is a research hotspot.
What is Bladder Cancer?
Bladder cancer is the general term for cancer that occurs in the bladder tissue[2]. And it is the 9th common malignant tumor and worldwide 13th cancer cause death[1]. The typical symptoms of bladder cancer include blood in the urine, pain with urination, and low back pain, etc. Bladder cancer tends to occur in the elder (most often between 65 and 84 years of age), and males have a higher risk of bladder cancer than females[2].
Causes of bladder cancer include cigarettes, family history, sequelae of radiation therapy and chemicals of exposure. The type of bladder cancer include transitional cell carcinoma, squamous cell carcinoma, adenocarcinoma and small cell bladder cancer. Transitional cell carcinoma (also called urothelial bladder cancer), is the most common type, while others are rare. And the therapy methods of bladder cancer mainly depend on surgery, radiation therapy, chemotherapy, or immunotherapy[2].
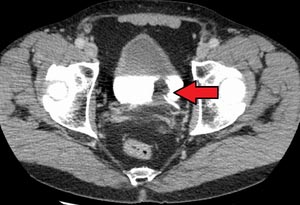
Figure.1 Transitional cell carcinoma of the bladder[2].
Application of Mouse Model in the Research of Bladder Cancer
The bladder cancer mouse model is a valuable tool for the in vivo research of bladder cancer. The application of the mouse bladder cancer model includes finding lineage relationships and cell of origin of bladder cancer, deconstructing the molecular mechanism of bladder cancer, analyzing the risk factor of bladder cancer and preclinical analyses of novel therapies[3].
For example, Erman et al. analyzed the early pathological process of bladder cancer by implanting exogenous cell lines into mice and analyzing the process of cancer cells invading the bladder epithelium. Throughout the early stages of bladder cancer, cancer cells first attach to the epithelium, then migrate to the basal layer and finally complete the connection with normal tissue cells and finally form tumors[4].
The methods for establishing a mouse bladder cancer model include exogenous transplantation, carcinogen treatment and genetically engineered. The models based on exogenous transplantation are called Non-autochthonous models. The grafts can be derived from cell lines or xenogeneic patients. The establishment of autochthonous carcinogen-based models is to treat mice by adding carcinogens (the most commonly used is BBN) in drinking water. Autochthonous genetically-engineered mouse (GEM) models include transgenic models, germline models and conditional alleles models[3]. The details are shown in Table.1.
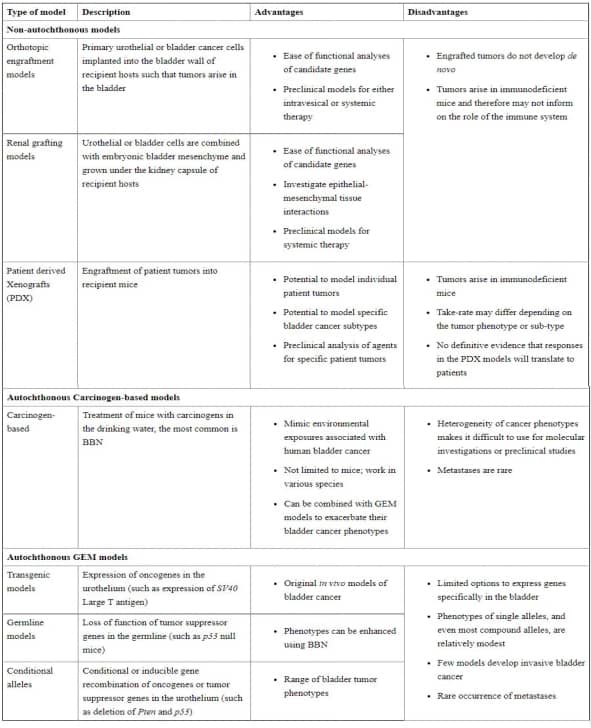
Table.1 mouse model of bladder cancers[3].
Mouse Bladder Cancer Cell Lines and culture protocol
Mouse bladder cancer cell lines are wildly used in the research of bladder cancer. It can be used for in vitro experiments of bladder cancer or as an exogenous graft for mouse bladder cancer models.
The cell lines widely used in research are mainly MB49 and MBT-2.
MB49 is developed from C57BL/6 bladder tumor induced by 7,12-dimethylenzanthacene[5]. Based on MB49, Lodillinsky et al. developed MB49-I invasive bladder tumor cell line. They subcutaneous inoculate MB49 in C57Bl/6J male mice and carry out 13 times in vivo passages of the primary tumor, and then obtain MB49-I[6].
Besides, MBT-2 was obtained from C3H/He mice treated with N-[4-(5-nitro-2-furyl)-2-thiazolyl] formamide (FANFT) in 1982[7]. But this cell line was found replicating type C retrovirus contamination in 2000[8].
The culture methods of these mouse bladder cancer cell lines are almost the same. MB49 and MBT-2 are both cultured at 37℃ 5% CO2 in RPMI-1640 with 10% FBS, 1% penicillin/streptomycin[9, 10].
Application of Mouse Bladder Cancer Cell Lines
Application of MB49 cell line in the research
Gilbertson et al. use MB49 to verify the sex differences of the mouse bladder cancer model[11]. They implant MB49 cell line into male and female C57BL/6 mice to establish mouse bladder cancer model. Compared with the female, male shows a greater growth of the tumor, and pregnancy does not affect female tumor growth. Besides, they treat MB49 cell line with human chorionic gonadotropin (hCG) and dihydrotestosterone in vitro. The proliferation of MB49 wasn’t influenced by hCG due to the absence of the receptor for gonadotropins, but dihydrotestosterone stimulates the growth of MB49 obviously.
MB49 is also applied to the research of bladder cancer immunotherapy. Vandeveer et al. transfect luciferase to MB49 (MB49luc) to identify the antitumor effect of an anti-programmed death-ligand 1(PD-L1) drug called avelumab[12]. The surface of MB49 has high Constitutive PD-L1 expression and avelumab can combine with PD-L1 to block the function of PD-L1. MB49luc are transplanted into C57BL/6 mice to establish bladder tumor model. They treat the mice with avelumab and identify that avelumab can slow down tumor growth and increase the survival rate. Besides, they identify that the treatment of avelumab with MB49 mouse bladder tumor model can generate a protective immunologic memory response to avoid secondary tumor formation by the secondary transplant of MB49.
Application of MBT-2 cell line in the research
Inactivated MBT-2 cell line can be an important component of bladder cancer vaccine. Huang et al. discover the specific tumor antigen from MBT-2 merged with the marine antimicrobial peptide (AMP) GE33 has the potential to become a cancer vaccine for immunotherapy[13]. The proliferation of MBT-2 can be inhibited by GE33 in vitro. And the immune effect induced by this vaccine can inhibit the tumor growth induced by MBT-2 and enhance the activation of T-cell receptors, cytotoxic T-cells, NK cells and the recruitment of monocytes, lymphocytes, T helper cells, and NK cells.
Ho et al. establish a growth-controllable bladder cancer mouse model based on the MBT-2 cell line [10]. The application of common bladder cancer mouse models based on MBT-2 is limited by the rapid death caused by uncontrollable rapid tumor growth. The researchers try to down-regulate the c-myc expression of MBT-2 through shRNA lentiviruses transduction to slow down the tumor growth. After the inhibition of c-myc expression, the growth of tumor-induced by MBT-2 is slowed down successfully in C3H/He mice. (Figure.2)
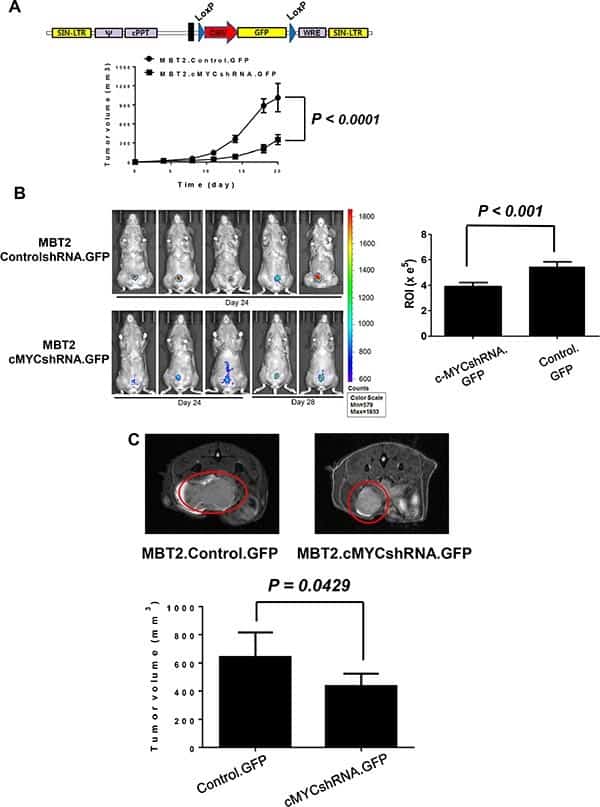
Figure.2 Growth-controllable bladder cancer mouse model based on the MBT-2 cell line.
Conclusion
Bladder cancer is a malignant disease with widespread impact. The research of pathological mechanisms, therapy methods, and clinical diagnosis has a lot of meaning. And mouse model and mouse bladder cancer cell lines are valuable tools for researchers to conduct research on bladder cancer.
Where to Get Mouse Bladder Cancer Cell Line for research?
AcceGen are committed to offering the most complete cell lines with the most favorable price. So far, we culture and provide commonly used bladder cancer mouse model cell lines MBT-2 and MB49. Other Mouse Bladder Cancer Cell Lines would also will available in AcceGen in the future.
It is our pleasure to help relative researches to move forward. All the products of AcceGen are strictly comply with international standards. For more detailed information, please visit our product portfolio or contact [email protected].
Reference
1. Dobruch J, Daneshmand S, Fisch M, Lotan Y, Noon AP, Resnick MJ, Shariat SF, Zlotta AR, Boorjian SA: Gender and Bladder Cancer: A Collaborative Review of Etiology, Biology, and Outcomes. Eur Urol 2016, 69:300-310.
2. https://enwikipediaorg/wiki/Bladder_cancer. wikipedia.
3. Kobayashi T, Owczarek TB, McKiernan JM, Abate-Shen C: Modelling bladder cancer in mice: opportunities and challenges. Nat Rev Cancer 2015, 15:42-54.
4. Erman A, Kamenšek U, Dragin Jerman U, Pavlin M, Čemažar M, Veranič P, Romih R: How Cancer Cells Invade Bladder Epithelium and Form Tumors: The Mouse Bladder Tumor Model as a Model of Tumor Recurrence in Patients. Int J Mol Sci 2021, 22.
5. Albertó M, Cuello HA, Gulino CA, Pifano M, Belgorosky D, Gabri MR, Eiján AM, Segatori VI: Expression of bladder cancer-associated glycans in murine tumor cell lines. Oncol Lett 2019, 17:3141-3150.
6. Lodillinsky C, Rodriguez V, Vauthay L, Sandes E, Casabé A, Eiján AM: Novel invasive orthotopic bladder cancer model with high cathepsin B activity resembling human bladder cancer. J Urol 2009, 182:749-755.
7. Mickey DD, Mickey GH, Murphy WM, Niell HB, Soloway MS: In vitro characterization of four N-[4-(5-nitro-2-furyl)-2-thiazolyl] formamide (FANFT) induced mouse bladder tumors. J Urol 1982, 127:1233-1237.
8. De Boer EC, Teppema JS, Steerenberg PA, De Jong WH: Retrovirus type C in the mouse bladder carcinoma cell line MBT-2. J Urol 2000, 163:1999-2001.
9. Chen F, Zhang G, Cao Y, Hessner MJ, See WA: MB49 murine urothelial carcinoma: molecular and phenotypic comparison to human cell lines as a model of the direct tumor response to bacillus Calmette-Guerin. J Urol 2009, 182:2932-2937.
10. Seo HK, Shin SP, Jung NR, Kwon WA, Jeong KC, Lee SJ: The establishment of a growth-controllable orthotopic bladder cancer model through the down-regulation of c-myc expression. Oncotarget 2017, 8:50500-50509.
11. White-Gilbertson S, Davis M, Voelkel-Johnson C, Kasman LM: Sex differences in the MB49 syngeneic, murine model of bladder cancer. Bladder (San Franc) 2016, 3.
12. Vandeveer AJ, Fallon JK, Tighe R, Sabzevari H, Schlom J, Greiner JW: Systemic Immunotherapy of Non-Muscle Invasive Mouse Bladder Cancer with Avelumab, an Anti-PD-L1 Immune Checkpoint Inhibitor. Cancer Immunol Res 2016, 4:452-462.
13. Huang HN, Rajanbabu V, Pan CY, Chan YL, Wu CJ, Chen JY: A cancer vaccine based on the marine antimicrobial peptide pardaxin (GE33) for control of bladder-associated tumors. Biomaterials 2013, 34:10151-10159.

Copyright - Unless otherwise stated all contents of this website are AcceGen™ All Rights Reserved – Full details of the use of materials on this site please refer to AcceGen Editorial Policy – Guest Posts are welcome, by submitting a guest post to AcceGen you are agree to the AcceGen Guest Post Agreement – Any concerns please contact [email protected]