- In-Stock Tumor Cell Lines
- Human Orbital Fibroblasts
- Human Microglia
- Human Pulmonary Alveolar Epithelial Cells
- Human Colonic Fibroblasts
- Human Type II Alveolar Epithelial Cells
- Human Valvular Interstitial Cells
- Human Thyroid Epithelial Cells
- C57BL/6 Mouse Dermal Fibroblasts
- Human Alveolar Macrophages
- Human Dermal Fibroblasts, Adult
- Human Lung Fibroblasts, Adult
- Human Retinal Muller Cells
- Human Articular Chondrocytes
- Human Retinal Pigment Epithelial Cells
- Human Pancreatic Islets of Langerhans Cells
- Human Kidney Podocyte Cells
- Human Renal Proximal Tubule Cells
Introduction
Nervous system diseases, especially central nervous system (CNS) diseases, have always been a thorny problem in clinical medicine and a research hotspot in basic medicine and physiology. As a key component of the CNS, microglia play key roles in CNS diseases, which is the research focus of CNS diseases. And microglia are active participants in brain function and brain dysfunction. Therefore, familiarity with BV-2, the most classic microglial cell model in vitro, will be of great help to researchers.
Overview: Nervous System and Glia
The nervous system is a highly complex key system that controls the actions, behaviors, physiological activities, and transmission of perceptual signals of the host. And it also plays a role as environmental adaptation regulators work in tandem with the endocrine system[1]. The nervous system includes the central nervous system (CNS) and the peripheral nervous system (PNS). The CNS is the most important part of the nervous system, consisting of the brain and spinal cord. And it is the central coordinator of the peripheral signal and the controller of the global physiological processes[2]. The PNS refers to the nerves and ganglia outside the brain and spinal cord, which play roles in the transmission of perceptual signals and regulation signals. And it consists of the somatic nervous system and the autonomic nervous system[3].
Glial cells (also called neuroglia) are the maintainer and protector of global physiological homeostasis and the nervous system[4; 5]. Glial cells can be divided into macroglia and microglia. Macroglia mainly include astrocytes (CNS), oligodendrocytes (CNS), ependymal cells (CNS), radial glia (CNS), Schwann cells (PNS), satellite cells (PNS), and enteric glia cells (PNS). These cells have different functions, such as blood supply regulation[6], providing insulation to the axon[7], the creation and secretion of cerebrospinal fluid[8], immunomodulation[9], the external chemical environment regulation of neurons[4], etc. Microglia are macrophages that can maintain and protect neurons located in the CNS[10]. Microglia are the primary immune cells of the CNS, which show an indispensable role in the first and main form of active immune defense[11].
Microglia and CNS-related pathology mechanism
Microglia are distributed in the whole brain and spinal cord, accounting for 10%~15% of the cells found in the brain[12]. Microglia are macrophages resident in the CNS and highly sensitive to lesions, which can timely and accurately remove plaques, damaged or useless neurons, and infectious pathogens[13; 14]. Besides, microglia also show key functions in extracellular signal transduction, which makes it become one of the core elements that must be paid attention to in CNS-related physiological and pathological processes[15]. Corresponding to the pathological changes of the CNS, microglia produce persistent morphological and physiological changes (Figure.1)[16].
In acute CNS damage, microglia show chemotaxis of the tissue damage site, prompting microglia to converge toward the damage site[17]. This protective effect of microglia depends on its tile-like network covering the entire neuropil under tightly regulated[18]. As immune cells (macrophages), microglia can secrete cytokine to involve in the inflammatory immune response of the CNS[19]. And microglia phagocytosis disorder is the cause of some CNS diseases[20]. Besides, microglia can be identified as direct cause of primary microgliopathies. The pathological mechanism is usually the physiological or morphological abnormality of microglia caused by a mutation[21].
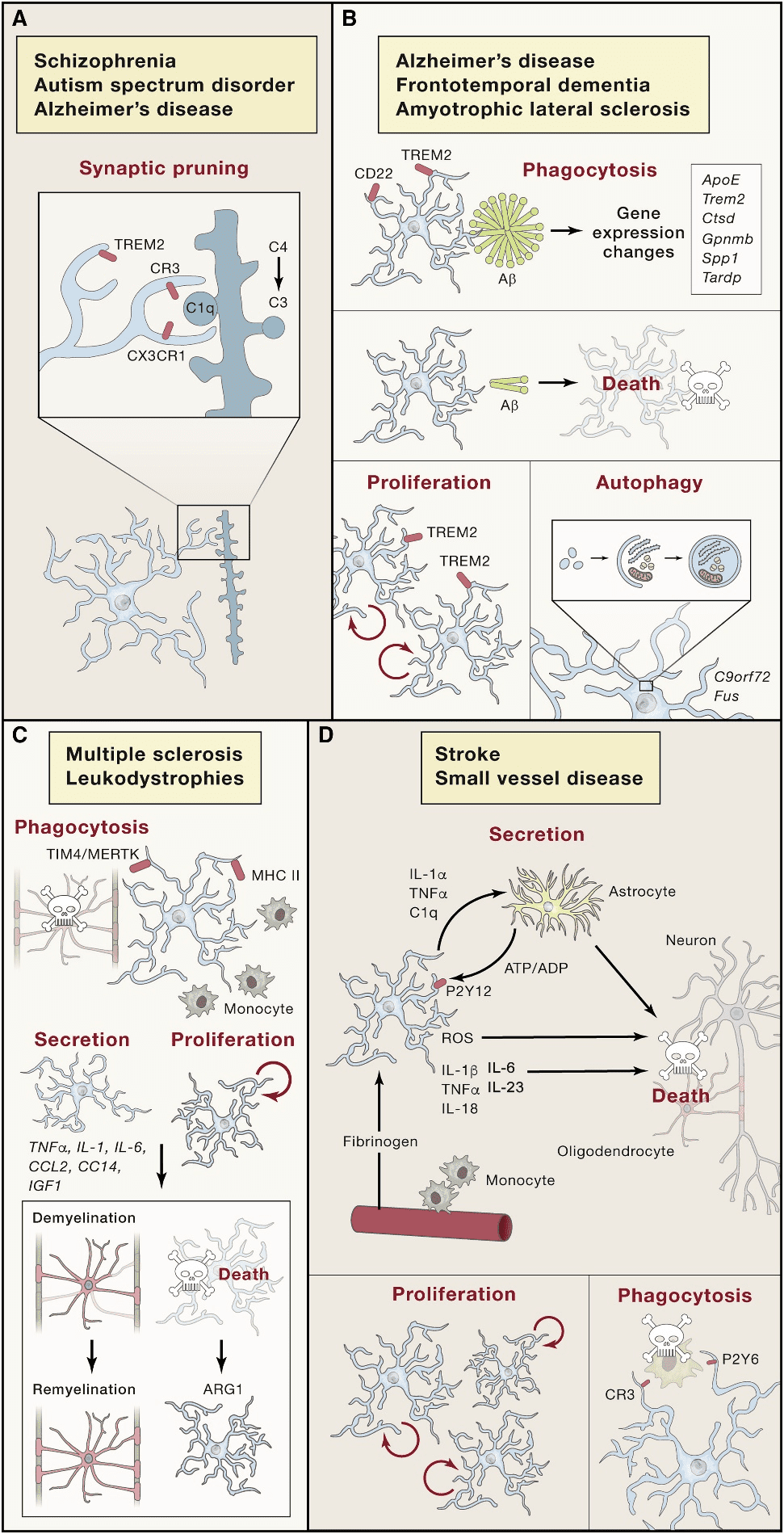
Figure.1[16] The form changes of microglia in the pathological processes of the CNS
Immortalized Microglia In Vitro Model: BV-2 Cell Lines
Microglia in vitro models are essential tools for the research of physiological functions and diseases of the CNS, such as peripheral macrophage-induced microglia, primary microglia, immortalized cell lines, pluripotent stem cell-derived microglia, and cerebral organoids[22]. Immortalized microglial cell lines are the most commonly used thanks to their easy cultivation and cheap application. The construction methods of immortalized microglial cell lines mainly include oncogene or SV40 large T antigen transduction, and one of the most classical cell lines is murine cell line BV-2[23; 24].
Murine immortalized microglial cell line BV-2 has been established for several decades, which is one of the most classical and commonly used immortalized cell lines and in vitro microglia models. It is generated by transducing v-raf/v-myc oncogene into primary microglial cells by a retrovirus [24]. The culture methods of BV-2 are very convenient. The recommended culture methods of BV-2 are DMEM with 10% FBS, 1% P/S and 1.1% GlutaMax (Substitute of L-glutamine) and culture at 37℃ with 5% CO2. And the recommended culture density of BV-2 is not exceeding 5 × 105 cells/ml[25].
Application of BV-2: The mechanism of chronic pain
Chronic pain seriously affects the quality of daily life of patients, and neuroinflammation in the spinal cord is a major cause of chronic pain. Qian, et al. revealed the role of KATP channels in neuroinflammation and chronic pain, and BV-2 cell line is an in vitro model in this work[26]. In the beginning, they found the cromakalim-induced opening of KATP channels can alleviate postoperative pain through animal models. After RNA-seq analysis of Cromakalim treatment, they found that the differentially expressed genes were enriched in inflammation-related signal and upregulation of suppressor of cytokine signaling-3 (SOCS3). And then, based on BV-2 as an in vitro model and combined with an in vivo mouse model, they identified that the opening of KATP could activate Growth arrest-specific 6 (Gas6)/Axl signal to induce the expression of SOCS3 and then induced the inflammation tolerance to inhibit the neuroinflammation-induced chronic pain.
Conclusion
CNS-related diseases and physiological processes are a long-term hot area. Microglia play key roles in CNS-related physiological and pathological processes, which makes the number of publications rapidly climb year by year. And BV-2 cell line as a classical in vitro model of microglia provides a great help to the rapid progress of microglia-related fields in recent decades.
Where to Get BV-2 Cell Line for Your Research?
AcceGen cultures and provides BV-2 cell line isolated from C57BL/6 mouse brain and transformed by a recombinant retrovirus (v-raf/v-myc) for neurobiology research. To get more information, please refer to: Tumor Cell Lines.
It is our pleasure to help relative researches to move forward. All the products of AcceGen strictly comply with international standards. For more detailed information, please visit our product portfolio or contact [email protected].
References
[1] G.J. Tortora, Derrickson, B, Principles of Anatomy and Physiology, J. Wiley, 2016.
[2] Farlex Partner Medical Dictionary. Farlex (2012).
[3] D. Laight, Overview of peripheral nervous system pharmacology. Nurse Prescribing 11 (2013) 448-454.
[4] K.R. Jessen, and R. Mirsky, Glial cells in the enteric nervous system contain glial fibrillary acidic protein. Nature 286 (1980) 736-7.
[5] R.D. Fields, A. Araque, H. Johansen-Berg, S.S. Lim, G. Lynch, K.A. Nave, M. Nedergaard, R. Perez, T. Sejnowski, and H. Wake, Glial biology in learning and cognition. Neuroscientist 20 (2014) 426-31.
[6] S. N, Brain-scan mystery solved. Scientific American Mind Oct–Nov (5): 7 (2008).
[7] N. Baumann, and D. Pham-Dinh, Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol Rev 81 (2001) 871-927.
[8] C.B. Johansson, S. Momma, D.L. Clarke, M. Risling, U. Lendahl, and J. Frisén, Identification of a neural stem cell in the adult mammalian central nervous system. Cell 96 (1999) 25-34.
[9] K.R. Jessen, and R. Mirsky, The origin and development of glial cells in peripheral nerves. Nat Rev Neurosci 6 (2005) 671-82.
[10] A.S. Warden, C. Han, E. Hansen, S. Trescott, C. Nguyen, R. Kim, D. Schafer, A. Johnson, M. Wright, G. Ramirez, M. Lopez-Sanchez, and N.G. Coufal, Tools for studying human microglia: In Vitro and In Vivo Strategies. Brain Behav Immun (2022).
[11] A.J. Filiano, S.P. Gadani, and J. Kipnis, Interactions of innate and adaptive immunity in brain development and function. Brain Res 1617 (2015) 18-27.
[12] L.J. Lawson, V.H. Perry, and S. Gordon, Turnover of resident microglia in the normal adult mouse brain. Neuroscience 48 (1992) 405-15.
[13] J. Gehrmann, Y. Matsumoto, and G.W. Kreutzberg, Microglia: intrinsic immuneffector cell of the brain. Brain Res Brain Res Rev 20 (1995) 269-87.
[14] L. Dissing-Olesen, R. Ladeby, H.H. Nielsen, H. Toft-Hansen, I. Dalmau, and B. Finsen, Axonal lesion-induced microglial proliferation and microglial cluster formation in the mouse. Neuroscience 149 (2007) 112-22.
[15] F. Aloisi, Immune function of microglia. Glia 36 (2001) 165-79.
[16] M. Prinz, S. Jung, and J. Priller, Microglia Biology: One Century of Evolving Concepts. Cell 179 (2019) 292-311.
[17] D. Davalos, J. Grutzendler, G. Yang, J.V. Kim, Y. Zuo, S. Jung, D.R. Littman, M.L. Dustin, and W.B. Gan, ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci 8 (2005) 752-8.
[18] K. Askew, K. Li, A. Olmos-Alonso, F. Garcia-Moreno, Y. Liang, P. Richardson, T. Tipton, M.A. Chapman, K. Riecken, S. Beccari, A. Sierra, Z. Molnár, M.S. Cragg, O. Garaschuk, V.H. Perry, and D. Gomez-Nicola, Coupled Proliferation and Apoptosis Maintain the Rapid Turnover of Microglia in the Adult Brain. Cell Rep 18 (2017) 391-405.
[19] M.T. Heneka, M.P. Kummer, A. Stutz, A. Delekate, S. Schwartz, A. Vieira-Saecker, A. Griep, D. Axt, A. Remus, T.C. Tzeng, E. Gelpi, A. Halle, M. Korte, E. Latz, and D.T. Golenbock, NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature 493 (2013) 674-8.
[20] D.P. Schafer, C.T. Heller, G. Gunner, M. Heller, C. Gordon, T. Hammond, Y. Wolf, S. Jung, and B. Stevens, Microglia contribute to circuit defects in Mecp2 null mice independent of microglia-specific loss of Mecp2 expression. Elife 5 (2016).
[21] E. Mass, C.E. Jacome-Galarza, T. Blank, T. Lazarov, B.H. Durham, N. Ozkaya, A. Pastore, M. Schwabenland, Y.R. Chung, M.K. Rosenblum, M. Prinz, O. Abdel-Wahab, and F. Geissmann, A somatic mutation in erythro-myeloid progenitors causes neurodegenerative disease. Nature 549 (2017) 389-393.
[22] A.S. Warden, C. Han, E. Hansen, S. Trescott, C. Nguyen, R. Kim, D. Schafer, A. Johnson, M. Wright, G. Ramirez, M. Lopez-Sanchez, and N.G. Coufal, Tools for studying human microglia: In Vitro and In Vivo Strategies. Brain, Behavior, and Immunity (2022).
[23] N. Janabi, S. Peudenier, B. Héron, K.H. Ng, and M. Tardieu, Establishment of human microglial cell lines after transfection of primary cultures of embryonic microglial cells with the SV40 large T antigen. Neurosci Lett 195 (1995) 105-8.
[24] E. Blasi, R. Barluzzi, V. Bocchini, R. Mazzolla, and F. Bistoni, Immortalization of murine microglial cells by a v-raf/v-myc carrying retrovirus. J Neuroimmunol 27 (1990) 229-37.
[25] R.J. Horvath, N. Nutile-McMenemy, M.S. Alkaitis, and J.A. Deleo, Differential migration, LPS-induced cytokine, chemokine, and NO expression in immortalized BV-2 and HAPI cell lines and primary microglial cultures. J Neurochem 107 (2008) 557-69.
[26] C. Qian, Y. Fan, L. Zong, C. Miao, L.L. Ji, L. Wan, R. Jia, X. Qin, Y. Wang, Q. Wu, X.Y. Tao, L. Hao, L. Hu, and W.T. Liu, Opening K(ATP) channels induces inflammatory tolerance and prevents chronic pain. Brain Behav Immun 107 (2022) 76-86.

Copyright - Unless otherwise stated all contents of this website are AcceGen™ All Rights Reserved – Full details of the use of materials on this site please refer to AcceGen Editorial Policy – Guest Posts are welcome, by submitting a guest post to AcceGen you are agree to the AcceGen Guest Post Agreement – Any concerns please contact [email protected]