- In-Stock Tumor Cell Lines
- Human Orbital Fibroblasts
- Human Microglia
- Human Pulmonary Alveolar Epithelial Cells
- Human Colonic Fibroblasts
- Human Type II Alveolar Epithelial Cells
- Human Valvular Interstitial Cells
- Human Thyroid Epithelial Cells
- C57BL/6 Mouse Dermal Fibroblasts
- Human Alveolar Macrophages
- Human Dermal Fibroblasts, Adult
- Human Lung Fibroblasts, Adult
- Human Retinal Muller Cells
- Human Articular Chondrocytes
- Human Retinal Pigment Epithelial Cells
- Human Pancreatic Islets of Langerhans Cells
- Human Kidney Podocyte Cells
- Human Renal Proximal Tubule Cells
Immunotherapy is believed to be one of the most promising areas of cancer research and treatment in the 21st century. As it is known that the immune system defends the body against infections. Immunotherapy can boost or change how the immune system works so it can find and attack cancer cells.
Immunomodulatory regimens have been proven to produce fewer side effects than medication. For example, it is less likely to create resistance when treating microbial disease. Therefore, immunotherapy has attracted great interest from researchers, clinicians, and pharmaceutical companies, especially in terms of its promise to treat various cancers.
There are many types of immunotherapy, including monoclonal antibodies and tumor-agnostic treatments, oncolytic virus therapy, T-cell therapy, Cancer vaccines, etc.
T cells, or T lymphocytes, play a key role in cell-mediated immunity. T cells can recognize, track, and fight against foreign particles in the body. CAR-T Cell Therapy takes advantage of this feature of T cells to target and to destroy cancer cells more effectively.
What is CAR-T Cell Therapy?
Chimeric antigen receptor (CAR) T Cell Therapy is a type of immunotherapy that genetically engineer patient’s T cells in the laboratory so that they can track and destroy cancer cells. T cells are modified with a viral vector to express an artificial or chimeric receptor for a particular cancer-associated antigen, such as CD19. Chimeric Antigen Receptor (CAR) is the receptor expressed by genetically engineered T cells on their membrane. The CAR on the cell’s surface is composed of fragments, or domains, of synthetic antibodies. Chimeric Antigen Receptor counts with an external target-binding domain designed to recognize a specific tumor antigen and an internal activation domain responsible for activating the T cell when the CAR-T binds its target. The domains that are used can affect how well the receptor recognizes or binds to the antigen on the tumor cell. Each CAR is specially made for certain cancer’s antigens as different cancers have different antigens.
CAR T-cell therapy is sometimes talked about as gene or cell therapy, or a type of immune effect cell therapy. It has been used to cure some hematological malignancies, such as acute lymphoblastic leukemia (ALL), chronic lymphocytic leukemia (CLL), multiple myeloma, and lymphoma. And it is still being developed to treat other cancer types, like melanoma, breast cancer, and sarcoma.
How it works?
CAR-T cell production is complex. The whole procedure takes several weeks, and also involves the cooperation of multiple organizations.
The whole process involves the following steps:
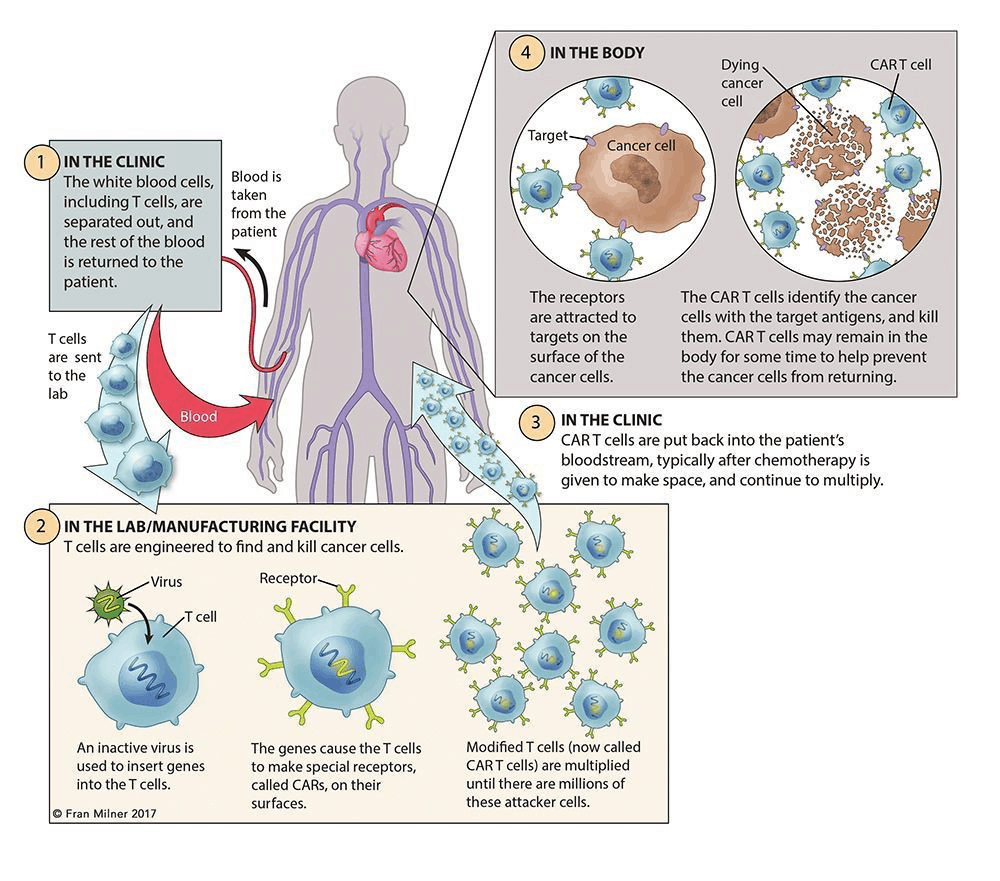
First in the hospital, collect T cells from the patient’s blood through phlebotomy or leukapheresis. Then in the following process of apheresis, granulocyte colony stimulating factor would be excluded, as it might disrupt T-cell proliferation and responsiveness.
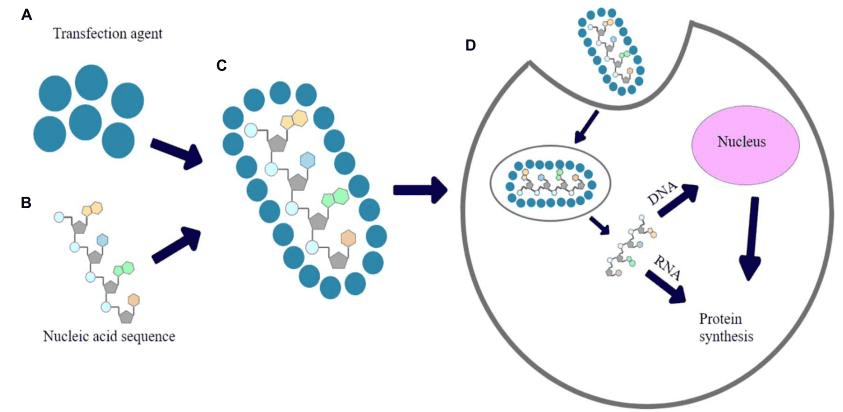
Then the CAR construct design could be done in hospital, academic institution, or manufacturer. Once it’s done and virus particles are ready prepared, transfect isolated T cells with a CAR viral (retroviral or lentiviral) or nonviral vector, where artificially insert a section of genome DNA.
Subsequent ex vivo expansion and purification are the critical steps, which determine the efficacy of this novel adoptive immunotherapy.
The final step of CAR-T cell production is to test the cell quality and sterility, which may take 2-4 weeks.
Limitations and the Future of CAR T-Cell Therapy
CAR-T cell therapy has shown its bright prospects as a cancer treatment. However, it is not affordable and effective for every patient. The whole process involves the cooperation of multiple organizations. Not only the fees for hospitals and academic institutions, the sample transport, analysis and CAR construct design, etc. are also the factors that affect the cost.
Furthermore, failures and relapses are inevitable in most cancer treatments. CAR T-cell Therapy is no exception. Therefore, track and record of CAR T cells in patients are necessary for the further improvement of CAR T-Cell therapy. Much more experimental researches are also essential for the development of this promising immune-oncology research/therapeutics.
How can AcceGen help you?
AcceGen proudly develops innovative technologies for life-science industry. In this service, we provide research-used only reagents for T cell transfection and culture. We provide products in a large range of categories to meet your specific needs. Our experienced team can always come up with professional services tailored to your needs. Please feel free to contact us at 1-862-686-2696, or send an email to [email protected]. Your questions and requests will be answered by expert staff in AcceGen within 24 hours.

Copyright - Unless otherwise stated all contents of this website are AcceGen™ All Rights Reserved – Full details of the use of materials on this site please refer to AcceGen Editorial Policy – Guest Posts are welcome, by submitting a guest post to AcceGen you are agree to the AcceGen Guest Post Agreement – Any concerns please contact [email protected]