- In-Stock Tumor Cell Lines
- Human Orbital Fibroblasts
- Human Microglia
- Human Pulmonary Alveolar Epithelial Cells
- Human Colonic Fibroblasts
- Human Type II Alveolar Epithelial Cells
- Human Valvular Interstitial Cells
- Human Thyroid Epithelial Cells
- C57BL/6 Mouse Dermal Fibroblasts
- Human Alveolar Macrophages
- Human Dermal Fibroblasts, Adult
- Human Lung Fibroblasts, Adult
- Human Retinal Muller Cells
- Human Articular Chondrocytes
- Human Retinal Pigment Epithelial Cells
- Human Pancreatic Islets of Langerhans Cells
- Human Kidney Podocyte Cells
- Human Renal Proximal Tubule Cells
Bone and Bone Cancer
Bone
The bone is a rigid organ that protects other organs and supports the body[1, 2]. There are about 300 bones in a newborn baby, and some of them will fuse during growth and development, and finally, 206 pieces are left in adulthood[3]. The main structures of bones include the outer cortex composed of dense bone[4], internal cortical bone[5], marrow[6], and cells[5]. Bones have a variety of functions, including a variety of mechanical functions[7], the Synthetic function of bone marrow (hematopoiesis)[8], and are involved in body metabolism (such as mineral and fat storage, pH balance regulation, detoxication, and calcium balance regulation). Besides, some diseases or injuries also occur in the bones, such as fractures, tumors, osteoporosis, etc.[9].

Figure.1 Microstructure of bone tissue
Tumor and Bone Cancer
Bone has the possibility of tumorigenesis, which may affect the bones to a certain extent. Benign tumors are mostly primary tumors, including osteoma, osteoid osteoma, osteochondroma, osteoblastoma, enchondroma, giant-cell tumors of bone, etc.[10]. And malignant tumors or cancer can be divided into primary and secondary cancers. Primary cancers include osteosarcoma, chondrosarcoma, Ewing’s sarcoma, fibrosarcoma, etc., and osteosarcoma is the most common primary bone cancer[11, 12]. Secondary cancers in the bone are predominantly intraosseous metastases, and common ones include breast cancer, lung cancer, prostate cancer, thyroid cancer, and kidney cancer. Bone cancer can cause osteolysis or sclerosis, osteolysis leads to decreased mechanical rigidity of bones, and sclerosis may lead to abnormal body support of bones. And cancers that involve the bone marrow can lead to leukemia or multiple myeloma, affecting blood production and the immune system[13].
Osteosarcoma and Bone Cancer Cell lines
Osteosarcomas are sourced from primitive mesenchymal cells mostly from bone and rarely originate from soft tissue[14]. Osteosarcoma that is not detected and treated in time has a rapid progression and multiple lung metastases (Cause of death in up to 90% of osteosarcoma patients), which will make clinical treatment very difficult[15]. Osteosarcomas are more common in adolescents, and the incidence in males is higher, 1.4 times that of females[16]. High incidence in adolescents implies the etiology of osteosarcomas may be related to rapid bone proliferation during adolescent growth and development. And the clinical treatment methods of osteosarcomas mainly depend on surgery, radiotherapy, polychemotherapy, and immunomodulation[14].
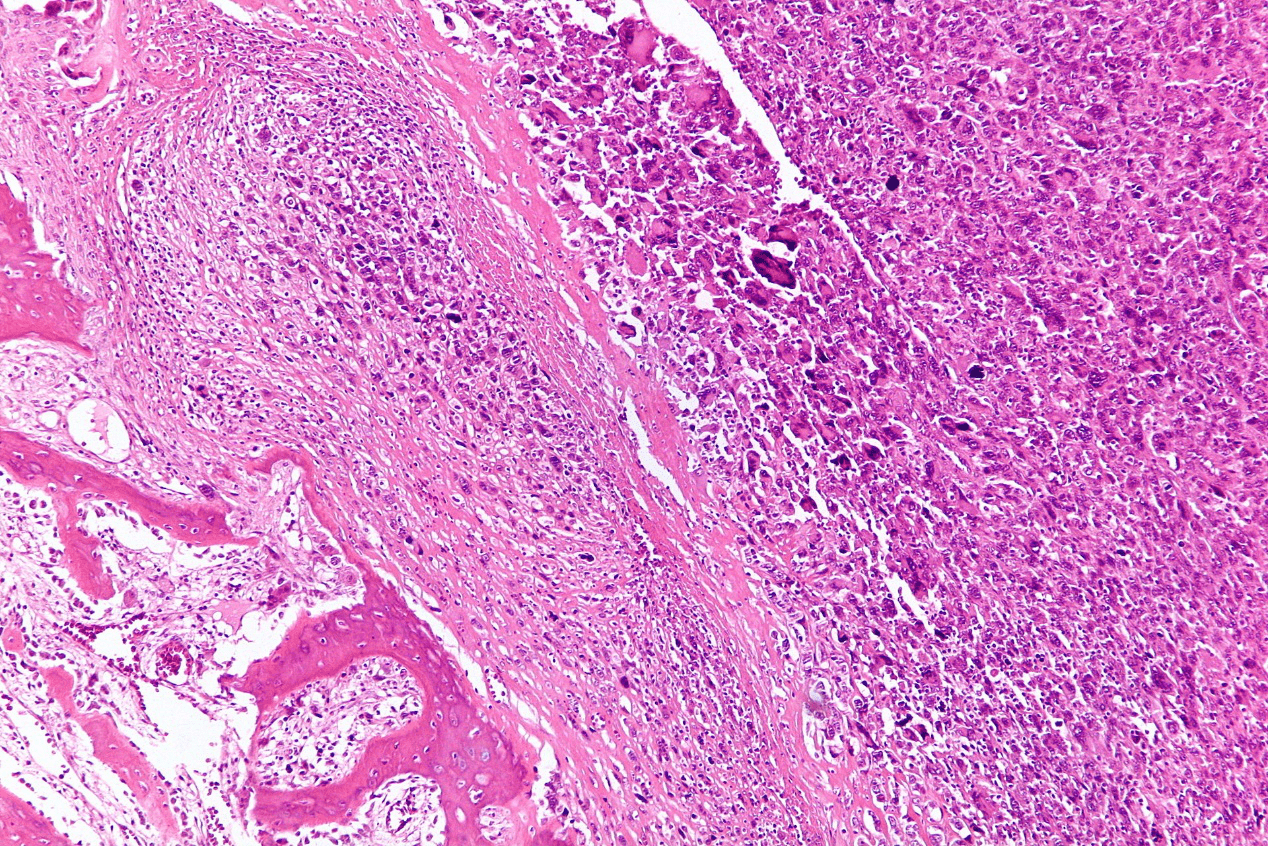
Figure.2 Osteosarcoma pathological section
Given the representation of osteosarcoma in primary bone cancers, osteosarcomas cell lines become one of the most common cell models in bone cancer research. Common osteosarcoma cell lines include MG-63, SAOS-2, HOS TE-85, U-2 OS, etc.[17], and MG63 and SAOS-2 will be highlighted here. As one of the standard cell models for bone research, MG-63 has been widely used for many years. Its physiological structure and physiological activity mechanism are highly similar to osteoblasts, and it has a relatively stable phenotype[18]. Similar to MG-63, SAOS-2 is another widely used cell line with several osteoblastic features and has higher alkaline phosphatase activity than other osteosarcomas cell lines[19, 20].
The culture methods of MG-63 and SAOS-2 are very similar and will be briefly introduced here[17]. The cells can be cultured in DMEM with 10% FBS or McCoy’s 5a medium with 15% FBS. The culture medium needs to add forty IU/ml of penicillin/streptomycin and avoid any stimulatory supplements or vitamins. It is recommended to use 37℃, 5% CO2 of the standard environment for the cultivation environment of the cells.
Application of Bone Cancer Cell lines
In view of the majority status of osteosarcoma in primary bone cancer, the application of bone cancer cell lines will be introduced here with osteosarcoma cell lines MG63 and SAOS-2.
MG-63
MG63 has high osteoblastic properties, so it is widely used in bone and bone cancer-related research. Yu, et al. used MG-63 as an osteoblast model to explore the apoptosis mechanism of osteoblasts during mechanical stretching[21]. They treated MG-63 with periostin and are subject to cyclic mechanical stretching. They found that MG-63 increased the number of apoptotic cells and cPARP expression after mechanical stretching, indicating that mechanical stretching can induce apoptosis of MG-63. And this apoptosis of MG-63 induced by mechanical stretching can be avoided by periostin through TGF-β signal. In summary, high-level mechanical stretch induces osteoblast apoptosis, and periostin can protect osteoblasts in this process. Besides, in the research on bone cancer, Bazavar et al. used MG-63 to analyze the mechanism of chemotherapeutic drug resistance in the treatment of bone cancer[22]. They found that microRNA-192 enhances the sensitivity of methotrexate drugs to osteosarcoma cancer cells, providing a reference for solving methotrexate drug resistance in clinical treatment.
SAOS-2
Similar to MG-63, SAOS-2 also possesses many properties of osteoblasts, making it one of the most commonly used models of osteoblasts. Lei, et al. used SAOS-2 to explore the mechanism of palmitic acid (PA)-induced osteoblast apoptosis[23]. PA is one of the most common long-chain saturated fatty acids in food. They treated SAOS-2 with PA and conducted a series of functional experiments. They find that after the treatment of PA, the SAOS-2 cell line produces stress of ER signaling and activation of the autophagy machinery, and eventually induces cell apoptosis. In conclusion, PA can induce osteoblast apoptosis via ER stress and autophagy.
Conclusion
Osteosarcoma predominates in primary bone cancers, so osteosarcoma cell lines have become a relatively widely used cell line among bone cancer cell lines. Osteosarcoma cell lines with osteoblastic properties are particularly favored by researchers because of their ease of culture and excellent cellular properties and are extremely valuable research tools.
Where to get Bone Cancer Cell Lines for research?
AcceGen provides sufficient bone cancer cell lines covering a wide range of bone cancer types, including Human primary osteogenic sarcoma cell lines (human osteosarcoma cell lines) – Saos-2, MG-63, 143B, SJSA-1, MNNG/HOS Cl #5, KHOS-NP, KHOS-312H, KHOS-240S, G-292 clone A141B1, cell line derived from Shwachman-Diamond syndrome – ROV S, human Ewing sarcoma cell lines – RD-ES, CADO-ES1, human neuroblastoma bone marrow metastasis cell line – LA-N-1, human osteoid osteoma cell line – HS903.T, human extraskeletal myxoid chondrosarcoma cell line – H-EMC-SS, and cell lines derived from Congenital pure red cell aplasia (Blackfan-Diamond anemia) – CM-S/un, CM S/Tum.
To get more information, please refer to: Human Bone Cancer Cell Lines.
It is our pleasure to help relative researches to move forward. All the products of AcceGen are strictly comply with international standards. For more detailed information, please visit our product portfolio or contact [email protected].
References
1. Lee CA, Einhorn TA: Chapter 1 – The Bone Organ System: Form and Function. In Osteoporosis (Second Edition). Edited by Marcus R, Feldman D, Kelsey J. San Diego: Academic Press; 2001: 3-20
2. de Buffrénil VdR, Armand J; Zylberberg, Louise; Padian, Kevin; Laurin, Michel; Quilhac, Alexandra: Vertebrate skeletal histology and paleohistology. Boca Raton, FL: CRC Press; 2021.
3. How Many Bones Does a Baby Have and Why Do Adults Have Fewer? Healthline.
4. Structure of Bone. https://flexbooksck12org/cbook/ck-12-college-human-biology-flexbook-20/section/134/primary/lesson/structure-of-bone-chumbio/.
5. Deakin BYea: Wheater’s functional histology : a text and colour atlas (5th ed.). Churchill Livingstone/Elsevier; 2006.
6. Barnes-Svarney PLS, Thomas E: The Handy Anatomy Answer Book : Includes Physiology. Detroit: Visible Ink Press; 2016.
7. Schmidt-Nielsen K: Scaling: Why Is Animal Size So Important? Cambridge Cambridge University Press; 1984.
8. Fernández KS, de Alarcón PA: Development of the hematopoietic system and disorders of hematopoiesis that present during infancy and early childhood. Pediatr Clin North Am 2013, 60:1273-1289.
9. Bone Diseases. Encyclopædia Britannica, Inc 27 August 2021.
10. Benign Bone Tumours. Cleveland Clinic 2017.
11. Jeon DG, Song WS, Kong CB, Kim JR, Lee SY: MFH of bone and osteosarcoma show similar survival and chemosensitivity. Clin Orthop Relat Res 2011, 469:584-590.
12. Ottaviani G, Jaffe N: The epidemiology of osteosarcoma. Cancer Treat Res 2009, 152:3-13.
13. Davidson SC, Nicki R.; Walker, Brian R.; Ralston, Stuart H.: Davidson’s principles and practice of medicine. Edinburgh: Churchill Livingstone/Elsevier.
14. Ritter J, Bielack SS: Osteosarcoma. Ann Oncol 2010, 21 Suppl 7:vii320-325.
15. C.D.M. F, K.K. U, F M: Pathology and genetics of tumours of soft tissue and bone. In World Health Organization Classification of Tumours. Lyon, France: IARC Press; 2002
16. Stiller CA, Bielack SS, Jundt G, Steliarova-Foucher E: Bone tumours in European children and adolescents, 1978-1997. Report from the Automated Childhood Cancer Information System project. Eur J Cancer 2006, 42:2124-2135.
17. Pautke C, Schieker M, Tischer T, Kolk A, Neth P, Mutschler W, Milz S: Characterization of osteosarcoma cell lines MG-63, Saos-2 and U-2 OS in comparison to human osteoblasts. Anticancer Res 2004, 24:3743-3748.
18. Staehlke S, Rebl H, Nebe B: Phenotypic stability of the human MG-63 osteoblastic cell line at different passages. Cell Biol Int 2019, 43:22-32.
19. Murray E, Provvedini D, Curran D, Catherwood B, Sussman H, Manolagas S: Characterization of a human osteoblastic osteosarcoma cell line (SAOS-2) with high bone alkaline phosphatase activity. J Bone Miner Res 1987, 2:231-238.
20. Rodan SB, Imai Y, Thiede MA, Wesolowski G, Thompson D, Bar-Shavit Z, Shull S, Mann K, Rodan GA: Characterization of a human osteosarcoma cell line (Saos-2) with osteoblastic properties. Cancer Res 1987, 47:4961-4966.
21. Yu KW, Yao CC, Jeng JH, Shieh HY, Chen YJ: Periostin inhibits mechanical stretch-induced apoptosis in osteoblast-like MG-63 cells. J Formos Med Assoc 2018, 117:292-300.
22. Bazavar M, Fazli J, Valizadeh A, Ma B, Mohammadi E, Asemi Z, Alemi F, Maleki M, Xing S, Yousefi B: miR-192 enhances sensitivity of methotrexate drug to MG-63 osteosarcoma cancer cells. Pathol Res Pract 2020, 216:153176.
23. Yang L, Guan G, Lei L, Lv Q, Liu S, Zhan X, Jiang Z, Gu X: Palmitic acid induces human osteoblast-like Saos-2 cell apoptosis via endoplasmic reticulum stress and autophagy. Cell Stress Chaperones 2018, 23:1283-1294.

Copyright - Unless otherwise stated all contents of this website are AcceGen™ All Rights Reserved – Full details of the use of materials on this site please refer to AcceGen Editorial Policy – Guest Posts are welcome, by submitting a guest post to AcceGen you are agree to the AcceGen Guest Post Agreement – Any concerns please contact [email protected]