- In-Stock Tumor Cell Lines
- Human Orbital Fibroblasts
- Human Microglia
- Human Pulmonary Alveolar Epithelial Cells
- Human Colonic Fibroblasts
- Human Type II Alveolar Epithelial Cells
- Human Valvular Interstitial Cells
- Human Thyroid Epithelial Cells
- C57BL/6 Mouse Dermal Fibroblasts
- Human Alveolar Macrophages
- Human Dermal Fibroblasts, Adult
- Human Lung Fibroblasts, Adult
- Human Retinal Muller Cells
- Human Articular Chondrocytes
- Human Retinal Pigment Epithelial Cells
- Human Pancreatic Islets of Langerhans Cells
- Human Kidney Podocyte Cells
- Human Renal Proximal Tubule Cells
Astrocytes
Astrocytes are named for their irregular star shape. They are the most abundant, extensively distributed, and largest group of neuroglia, accounting for almost half of all neuroglia. Astrocytes can develop and differentiate from embryonic neuroepithelial cells in the ventricular zone (VZ). Astrocytes can also develop from immature cells in the subventricular zone (SVZ) that migrate to gray or white matter during the perinatal stage.
Morphology and specific markers of astrocytes
Astrocytes are 9-10 µm in diameter and have numerous radial projections from their cell bodies. The nucleus is round or ovoid, with a small amount of heterochromatin attached to the inner surface of the nuclear membrane and an inconspicuous nucleolus.
GFAP is an intermediate filament protein specifically present in astrocytes, and its expression gradually increases during the development, differentiation and maturation of astrocytes. It is a specific marker for astrocytes. In addition to GFAP, Ca2+-binding protein S100β, GLAST, glutamate transporter-1 (GLT-1), glutamine synthetase (GS), and aldehyde dehydrogenase 1 family member L1 protein (ALDH1L1) are all specific indicators for astrocytes [1].
Functions of astrocytes
Astrocytes functions are ranging from expression of numerous functionally essential membrane proteins, forming gap junctions between individuals [2], physically supporting of neurons [3], forming tripartite synapse structures together with neurons [4], and contributing to formation of the blood-brain barrier (BBB), to maintenance of neuronal energy metabolism, regulating neuronal development, participation in other physiological functions such as maintaining water homeostasis and regulating the extracellular microenvironment in the brain [5].
Furthermore, astrocytes also resist oxidative stress [2], store energy in the form of glycogen, form scar and repair tissues, regulate synapse formation, function, activity, and remodeling through the release of a variety of cytokines, neurotrophic factors, and gliotransmitters, and regulate neuronal signaling and behavior [6].
Astrocytes and pathologic injuries
Astrocytes are activated in response to a range of harmful stimuli or throughout the aging process. Activated astrocytes undergo a series of changes, including an increase in the size of reactive astrocyte cytosol and protrusions, an increase in the content of GFAP accompanied by a variety of changes in gene expression, and an increase in the number of cells that migrate and number of cells, and so on [7]. These alterations are referred to as astrogliosis.
After suffering from mechanical injury, gliosis is the most prominent manifestation of astrocytes. On the one hand, gliosis of astrocytes forms a glial scar that protects the damaged area and prevents the spread of the injury. On the other hand, the prompt and robust nature of gliotic responses results in the persistence of the glial scar within the injured space impeding timely degradation, thus affecting the function of the neighboring neural pathways, regeneration of axons and remyelination. Moreover, glial scarring is also involved in seizures.
Under physiological conditions, astrocytes sense the osmolarity of their extracellular environment and regulate their own cell volume. However, in cases of pathological injury, this volume-regulating mechanism is inhibited, resulting in cerebral edema along with alterations in extracellular fluid osmolarity, acidosis, and ammonia toxicity. When the osmotic pressure of the cerebrospinal fluid decreases due to excessive water intake, water molecules pass through AQP1, AQP4, and AQP5 which are localized on the astrocytic membrane, and enter the cell in response to the osmotic pressure gradient, causing edema in the astrocyte. The edema of astrocytes leads to a decrease in extracellular space and an increase in the levels of K+ and glutamate in the cerebrospinal fluid; the end-foot of the edematous astrocytes compresses the microvessels, causing further cerebral ischemia and aggravating cerebral edema [5].
Astrocytes are involved in pathological injury processes such as cerebral hypoxia and ischemia and play a dual role therein. On the one hand, astrocytes play a protective role by balancing the K+ concentration in the extracellular fluid, uptake of glutamate in the extracellular fluid, and synthesizing and releasing some neurotrophic factors. On the other hand, severe ischemia causes astrocytes to swell, producing inflammatory factors and a large number of free radicals, which aggravates ischemic injury. At the same time, during ischemia, the high affinity glutamate transporters GLAST and GLT-1 in astrocytes reversed intracellular glutamate transport to the outside of the cell due to a decrease in membrane potential and disruption of the intra- and extracellular ionic gradient, which aggravated excitotoxic injury.
Astrocytes are also involved in the development and progression of degenerative diseases of the central nervous system such as Alzheimer’s disease, Parkinson’s disease, amyotrophic lateral sclerosis, and Alexander disease.
Astrocytes and Alzheimer’s disease
The pathogenesis of Alzheimer’s disease (AD) is still unknown, but three major pathological changes are currently accepted: (1) amyloid plaque; (2) neurofibrillary tangle (NFT); and (3) regional neuroinflammation, as well as neuronal and synaptic deficits resulting from these changes. The formation of amyloid plaques may be the fundamental pathologic alteration in AD, and its main components are Aβ (beta amyloid), astrocytes (Figure 1), and microglia [8].
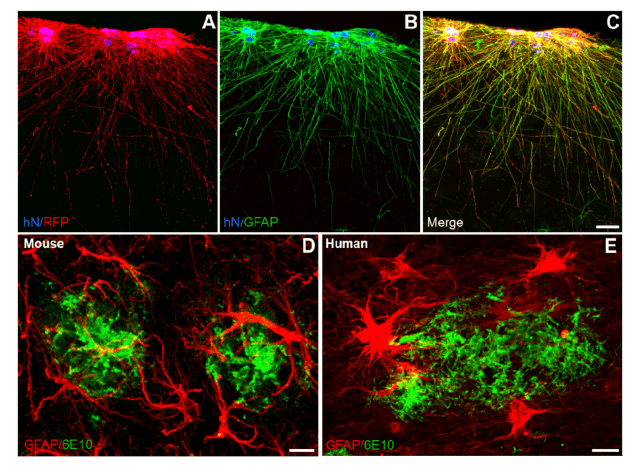
Figure 1. Astrocyte morphologies in healthy and in Alzheimer’s disease brains. (A–C) Human iPSC-derived astroglialprogenitors transplanted into the mouse brain (RFP, red) integrate in the cortex and develop into interlaminar astrocytesexpressing GFAP (green). hN: human Nuclei stains the nuclei of human cells. Scale bar: 25 µm. (D–E) Close interactionof both mouse and human astrocytes with β-amyloid plaques. GFAP-positive mouse or human astrocytes (red) around β-amyloid plaques (6E10, green) in the cortex of an APP/PS1 mouse (D) and in the entorhinal cortex of an Alzheimer´sdisease patient brain (E). Scale bars: 10 µm.
Astrocytes phagocytose Aβ-rich neuronal degradation products, leading to their accumulation within the cells. Under physiological conditions, there is a dynamic balance between the accumulation of Aβ in astrocytes and its clearance. Whereas in Alzheimer’s disease, the expression of AQP4 is down-regulated in astrocytes, resulting in the blockage of glial lymphatic reflux, and the clearance rate of Aβ is lower than the accumulation rate, allowing Aβ to activate the astrocytes and eventually form amyloid plaques.
Another characteristic pathology of AD is NFT. Tau is a very important microtubule-associated protein in neurons. Abnormal modification of Tau protein, especially overphosphorylation, leads to intracellular deposition of Tau protein and the formation of NFTs. Astrocytes release apolipoprotein E (ApoE), which binds specifically to Tau proteins and inhibits their phosphorylation and self-aggregation. ApoE gene counts in AD patients and the general population reveal that those who have the ApoEε4 gene, which does not bind Tau protein, are more likely to develop AD.
During the pathogenesis of AD, astrocytes secrete S100β, which promotes the accumulation of Tau proteins and the formation of NFTs. The abnormal accumulation of Aβ and the NFTs triggers a neuroinflammatory response, together with the release of inflammatory cytokines, which can in turn promote the formation of Aβ and NFTs. Aβ functions as an antigen and activates astrocytes, which not only phagocytose but also produce and release a variety of inflammatory cytokines that mediate neuronal damage and death, resulting in the pathologic damage of Alzheimer’s disease.
Conclusions
In this brief review, we give an overview of astrocytes in the aspects of their origin, morphology, specific markers, main functions, molecular and morphological characteristics in pathologic injuries and Alzheimer’s disease. We propose that astrocytes are the alternative targets should be focused on to fight both brain injuries and Alzheimer’s disease.
References
[1] L.C. Yu, Neurobiology., Peking University Press2012.
[2] H.T. Le, W.C. Sin, S. Lozinsky, J. Bechberger, J.L. Vega, X.Q. Guo, J.C. Sáez, C.C. Naus, Gap Junction Intercellular Communication Mediated by Connexin43 in Astrocytes Is Essential for Their Resistance to Oxidative Stress, Journal of Biological Chemistry 289(3) (2014) 1345-1354.
[3] G. Miller, The Dark Side of Glia, Science 308(5723) (2005) 4.
[4] G. Perea, M. Navarrete, A. Araque, Tripartite synapses: astrocytes process and control synaptic information, Trends in Neurosciences 32(8) (2009) 421-431.
[5] Z. Zhou, J. Zhan, Q. Cai, F. Xu, R. Chai, K. Lam, Z. Luan, G. Zhou, S. Tsang, M. Kipp, W. Han, R. Zhang, A.C.H. Yu, The Water Transport System in Astrocytes–Aquaporins, Cells 11(16) (2022).
[6] M.D. Scofield, Exploring the Role of Astroglial Glutamate Release and Association With Synapses in Neuronal Function and Behavior, Biological Psychiatry 84(11) (2018) 778-786.
[7] K. Gao, C.R. Wang, F. Jiang, A.Y.K. Wong, N. Su, J.H. Jiang, R.C. Chai, G. Vatcher, J. Teng, J. Chen, Y.-W. Jiang, A.C.H. Yu, Traumatic scratch injury in astrocytes triggers calcium influx to activate the JNK/c-Jun/AP-1 pathway and switch on GFAP expression, Glia 61(12) (2013) 2063-2077.
[8] P. Preman, M. Alfonso-Triguero, E. Alberdi, A. Verkhratsky, A.M. Arranz, Astrocytes in Alzheimer’s Disease: Pathological Significance and Molecular Pathways, Cells 10(3) (2021).

Copyright - Unless otherwise stated all contents of this website are AcceGen™ All Rights Reserved – Full details of the use of materials on this site please refer to AcceGen Editorial Policy – Guest Posts are welcome, by submitting a guest post to AcceGen you are agree to the AcceGen Guest Post Agreement – Any concerns please contact [email protected]