- In-Stock Tumor Cell Lines
- Human Orbital Fibroblasts
- Human Microglia
- Human Pulmonary Alveolar Epithelial Cells
- Human Colonic Fibroblasts
- Human Type II Alveolar Epithelial Cells
- Human Valvular Interstitial Cells
- Human Thyroid Epithelial Cells
- C57BL/6 Mouse Dermal Fibroblasts
- Human Alveolar Macrophages
- Human Dermal Fibroblasts, Adult
- Human Lung Fibroblasts, Adult
- Human Retinal Muller Cells
- Human Articular Chondrocytes
- Human Retinal Pigment Epithelial Cells
- Human Pancreatic Islets of Langerhans Cells
- Human Kidney Podocyte Cells
- Human Renal Proximal Tubule Cells
The nose is the entrance of the respiratory tract. And Nasal cavity is the first barrier and line of defense of the respiratory system. Nasal epithelium and mucosa are physical and immune barrier between the internal and external environment of the host in the nasal cavity. Therefore, nasal epithelium and mucosa are popular areas for researchers.
Nasal cavity and Nasal mucosa
The nasal cavity is the uppermost part of the respiratory system. It is located behind the nose in the middle of the face. The nasal cavity can be divided into the respiratory segment and the olfactory segment. One of the functions of the nasal cavity is to receive air and humidity and heat it. Besides, the nasal cavity can filter out particles and dust in the inhaled air. Another function of the nasal cavity is to accommodate the ability to smell. As the entrance to the respiratory tract, the diseases of the nasal cavity include microbial (such as viral, bacterial and fungal) infections and tumors [1].
There is a mucosa system in the nasal cavity, and the nasal mucosa is also part of the respiratory mucosa. The function of the nasal mucosa is to filter air, which makes the nasal mucosa more prone to inflammation. The mucosal membrane of the meatuses on the floor of the nasal cavities and the various sinuses is thinner than the nasal conchae and the nasal septum, which makes it become one of the most commonly infected tissues. This inflammation can seriously affect the patient’s daily life [2].
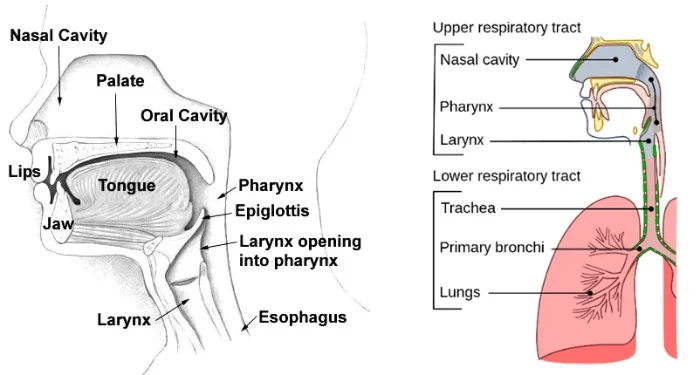
Figure.1.The structure of Nasal cavity and respiratory system
Nasal Epithelium、Mucosa and Nasal Epithelial Cells(NEC)Model
The nasal epithelium and mucosa system are important parts of the nasal cavity. The nasal epithelium and mucosa are interface between the internal and external environment. This system acts as a physical barrier as well as an immune barrier of the respiratory tract. The physical barrier is a mechanical barrier established by tightly connected epithelial cells. And the immune barrier refers to the innate immunity and acquired immunity of the nasal mucosa supported by cytokines and chemokines. Besides, chronic inflammation (such as chronic rhinosinusitis, CRS) may cause damage to the nasal epithelium and then induce epithelial–mesenchymal transition (EMT). And EMT may cause tissue fibrosis of the nasal epithelium [3].
Primary nasal epithelial cells and immortalized nasal epithelial cells are commonly used in vitro models. The advantage of primary nasal epithelial cells is the more reduced heredity and phenotype, but the isolation and culture methods of primary cells are difficult and the number of passages is limited. Correspondingly, immortalized nasal epithelial cell lines are easy to culture and have high reproducibility, but there may be genetic and phenotypic differences relative to primary cells [4].
Isolation and Culture Protocol of Human Nasal Epithelial Cells(hNEC) based on Air-Liquid Interface (ALI) model
Human nasal epithelial cells (hNEC) are a common human-derived in vitro model used for research and construction of the nasal cavity. Traditional primary and immortalized hNEC in vitro models are similar to conventional cell separation and culture methods. And traditional models are easy to operate and enough for regular in vitro functional experiments. Recently, the air-liquid interface (ALI) model system become a highly valued method for the in vitro culture of hNEC. In the culture of hNEC ALI in vitro model, hNEC can differentiate into heterogeneous cell populations to reproduce the physiological structure and complete physiological function of the nasal epithelium system in vivo. Despite the simpler manipulation, this feat is difficult to be achieved by traditional hNEC in vitro models [5].
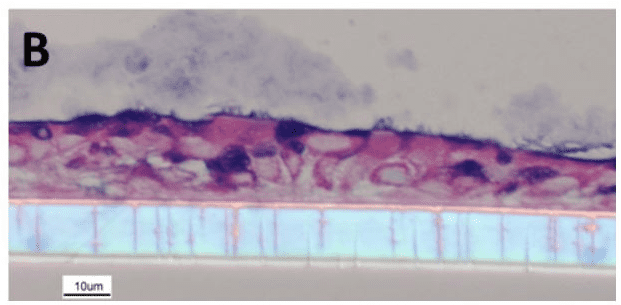
Figure.2 Re-differentiated human NEC culture. The culture shows tissue structures similar to in vivo, including cilia, cup cells and mucus produced by cup cells.
The main steps of separation and culture of hNEC based on the ALI model include biopsy sampling, expansion, seeding, differentiation, and interface establishment. Firstly, the tissue of the subject is sampled by sterile thermoplastic curette or interdental brush and collected. The biopsy tissue is cultured and amplification in a culture plate 2-4 days at 37℃, 5% CO2 after pretreatment. If the colonies can be visible by day 4, then the isolation of the HNEC is a success. And then, the hNEC can be seeded onto 12 mm polyester transwell inserts to finish the differentiation. And finally, the ALI in vitro hNEC model is successfully established [5, 6].
Applications of Human Nasal Epithelial Cells
The nasal epithelial cells that have completed differentiation and formed the epithelial mucosa system can obtain their complete physiological functions. Therefore, the application of the ALI hNEC in vitro model is more common than the traditional hNEC in vitro model in recent publications. And the application of traditional hNEC in vitro models is mainly focusing on the study of molecular mechanisms that do not involve complex physiological functions. For example, Larsson, et al. use primary hNEC to analyze the molecular mechanisms of toll-like receptors (TLRs) signal in allergic rhinitis (AR) [7].
The application of hNEC in the exploration of nasal administration
hNEC can be used to explore the feasibility of nasal administration. Bequignon, et al. found the feasibility of the neonatal nasal administration of monoclonal antibodies (mAbs) and explored the mechanism of drug absorption through ALI hNEC in vitro model[8]. Neonatal Fc receptor (FcRn) is a transport protein expressed in neonatal hNEC. FcRn participates in the transportation and procession of IgG in hNEC. The researchers found that the expression level of FcRn increases with the differentiation of hNEC in the ALI model. And they located the main distribution area of FcRn as the apical part of the cytosol at the base of cilia and on the basolateral surface of ciliated cells. Furthermore, they found that mABs transcytosis can successfully occur in the ALI hNEC model. These results lay the foundation for neonatal mAbs nasal administration.
The application of hNEC in the research of SARS-CoV-2
hNEC ALI model also used into the research of the novel coronavirus SARS-CoV-2. Gamage, et al. use the hNEC ALI model to study the mechanism of SARS-CoV-2 infection and pathogenesis [9]. After in vitro infection experiment on the ALI hNEC model, they found that airway epithelium is the infection target of SARS-CoV-2. And the immune response of the airway epithelium and mucosa after SARS-CoV-2 infection is lower than the infection of influenza strain, H3N2. And then, they identified the CXCL10 as the only cytokine significantly induced upon infection of SARS-CoV-2 through the SARS-CoV-2 infection experiment on ALI hNEC model. Besides, they compare the infection process of the original strain and 382-nt deletion isolate lacking ORF8 (isolated in Singapore in March 2020) on the ALI hNEC model. These strains show similar viral kinetics and host transcriptional profiles and then verify ORF8 is a non-essential part of the pathogenic process of SARS-CoV-2.
Conclusion
Nasal epithelium is the first line of defense of the respiratory system with a high incidence of disease. The nasal epithelium and mucosal system are the two most important subsystems of the respiratory system. Human nasal epithelial cells and the ALI hNEC in vitro model are valuable tools in physiology and medical research. SARS-CoV-2 is spreading worldwide, and the respiratory system is the main target of the virus. This makes the nasal epithelium a more important research object than ever before.
Where to get Human Nasal Epithelial Cells
AcceGen provides the most authentic Human Nasal Epithelial Cells and Immortalized Human Nasal Epithelial Cells to meet various research needs. Besides, AcceGen provides different types of epithelial cells from respiratory system. To get more information, please refer to: Respiratory System Primary Cells.
It is our pleasure to help relative researches to move forward. All the products of AcceGen are strictly comply with international standards. For more detailed information, please visit our product portfolio or contact inquiry@accegen.com.
Reference
1. cavity N: https://en.wikipedia.org/wiki/Nasal_cavity. wikipedia.
2. mucosa N: https://en.wikipedia.org/wiki/Nasal_mucosa. wikipedia.
3. Scherzad A, Hagen R, Hackenberg S: Current Understanding of Nasal Epithelial Cell Mis-Differentiation. J Inflamm Res 2019, 12:309-317.
4. Charles DD, Fisher JR, Hoskinson SM, Medina-Colorado AA, Shen YC, Chaaban MR, Widen SG, Eaves-Pyles TD, Maxwell CA, Miller AL, et al: Development of a Novel ex vivo Nasal Epithelial Cell Model Supporting Colonization With Human Nasal Microbiota. Frontiers in Cellular and Infection Microbiology 2019, 9.
5. Müller L, Brighton LE, Carson JL, Fischer WA, 2nd, Jaspers I: Culturing of human nasal epithelial cells at the air liquid interface. J Vis Exp 2013.
6. Manna V, Caradonna S: Isolation, expansion, differentiation, and histological processing of human nasal epithelial cells. STAR Protoc 2021, 2:100782.
7. Larsson O, Sunnergren O, Bachert C, Kumlien Georén S, Cardell LO: The SP-TLR axis, which locally primes the nasal mucosa, is impeded in patients with allergic rhinitis. Clin Transl Allergy 2021, 11:e12009.
8. Bequignon E, Dhommée C, Angely C, Thomas L, Bottier M, Escudier E, Isabey D, Coste A, Louis B, Papon JF, Gouilleux-Gruart V: FcRn-Dependent Transcytosis of Monoclonal Antibody in Human Nasal Epithelial Cells In Vitro: A Prerequisite for a New Delivery Route for Therapy? Int J Mol Sci 2019, 20.
9. Gamage AM, Tan KS, Chan WOY, Liu J, Tan CW, Ong YK, Thong M, Andiappan AK, Anderson DE, Wang Y, Wang LF: Infection of human Nasal Epithelial Cells with SARS-CoV-2 and a 382-nt deletion isolate lacking ORF8 reveals similar viral kinetics and host transcriptional profiles. PLoS Pathog 2020, 16:e1009130.

Copyright - Unless otherwise stated all contents of this website are AcceGen™ All Rights Reserved – Full details of the use of materials on this site please refer to AcceGen Editorial Policy – Guest Posts are welcome, by submitting a guest post to AcceGen you are agree to the AcceGen Guest Post Agreement – Any concerns please contact marketing@accegen.com