- In-Stock Tumor Cell Lines
- Human Orbital Fibroblasts
- Human Microglia
- Human Pulmonary Alveolar Epithelial Cells
- Human Colonic Fibroblasts
- Human Type II Alveolar Epithelial Cells
- Human Valvular Interstitial Cells
- Human Thyroid Epithelial Cells
- C57BL/6 Mouse Dermal Fibroblasts
- Human Alveolar Macrophages
- Human Dermal Fibroblasts, Adult
- Human Lung Fibroblasts, Adult
- Human Retinal Muller Cells
- Human Articular Chondrocytes
- Human Retinal Pigment Epithelial Cells
- Human Pancreatic Islets of Langerhans Cells
- Human Kidney Podocyte Cells
- Human Renal Proximal Tubule Cells
Introduction: Luciferase and Neuron
When luciferase binds to the target protein, its fluorescence signal can be measured very accurately, which makes it suitable for detecting the amount of cell signal in real-time. Neurons, the basic units of the brain and nervous system, are responsible for receiving sensory input from the outside world and sending movement to muscles. A large number of cell signaling molecules exist in it, which regulate many biological activities such as their differentiation, repair, and inflammation. Luciferase is increasingly used in the study of nerve cells, which brings important impetus to medical research on the brain.
Luciferase
Luciferase is a biological enzyme that occurs naturally in fireflies and luminescent marine and terrestrial microorganisms. When luciferase is fused to the target protein, its fluorescence signal can be measured very accurately using a luminometer[1].
Although several luciferases have been identified, only three types of luciferin are commercially available: firefly D-luciferin, coelenterazine, and Cypridina luciferin[2].
Renilla and Gaussia luciferase use coelenterazine as a substrate; Firefly luciferase reactions require adenosine triphosphate (ATP), O2, and Mg2+ in addition to the substrate. Cypridina uses its luciferin as a substrate. Luciferase is commonly used to report the expression level of proteins fused to it.[1].
These luciferases, particularly the eukaryotic firefly luciferase, have been commonly used as light probes in several biological experiments, including promoter activity assays[3], RNAi interference detection, Protein interaction, in vivo imaging and microbial detection. The introduction of luciferase and other light-emitting proteins as visual markers/reporters has drastically expanded the versatility of reporter gene technology. Expression of the luciferase gene fusion product confers on the target protein the ability to glow in the dark[4].
Luciferase can be expressed constitutively, to enable the noninvasive tracking of cells that express the enzyme (Figure.1). In this way, the survival and cell growth of transplanted cells can be monitored in vivo[5].
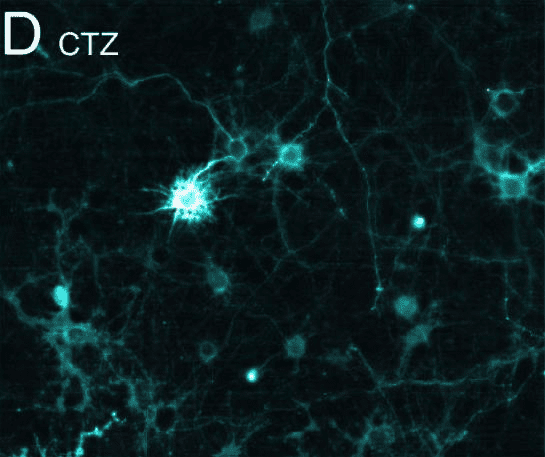
Figure.1 Bioluminescence elicits postsynaptic currents in vitro[6]
Neuron
Two primary cell types exist in the brain: nerve cells (Neurons) and glial cells.
Neurons are members of the class of cells most closely related to nervous system function. They are atoms of perception, memory, thought, and action, and thus the atoms of consciousness[7]. The term neuron was coined by the German neuroanatomist Wilhelm Waldeyer in 1891.
The human brain consists of more than 100 billion neurons. Information is processed in the brain as the form of electrical signals that are transmitted across the cell membrane of neurons and between neurons through contact points called synapses. Signal transmission between synapses depends on neurotransmitters. They are released from synaptic vesicles into synaptic spaces, where they can interact with receptors on target cells. Neurotransmitters play an essential role in the function of the nervous system. Neurons membranes contain various ion channels whose activity can alter electrical signals and thus change transmitted information. Neurons form highly interconnected complex networks in which different aspects of sensory information are processed and eventually transformed into meaningful behavior[8].
Typical neurons have a cell body containing one nucleus and two or more long fibers. Pulses are transmitted to the cell body along one or more fibers called dendrites. In the higher nervous system, only one fiber, the axon, transmits impulses from the cell body[9]. Fiber bundles from neurons are held together by myelin sheaths formed by glial cells to form nerve fibers (Figure.2). Nerve fibers are made up of the long projections of neurons and the glial cells that surround them. Its main function is to conduct nerve impulses. It can be divided into two types: medullated nerve fibers and unmedullated nerve fibers. Glial cells are the most abundant cells in the central nervous system[10]. In addition to providing support for the basic functions of the nervous system, it helps to maintain homeostasis and form myelin. The most notable glial cells include astrocytes, oligodendrocytes, Schwann cells, microglia, and ependymal cells.
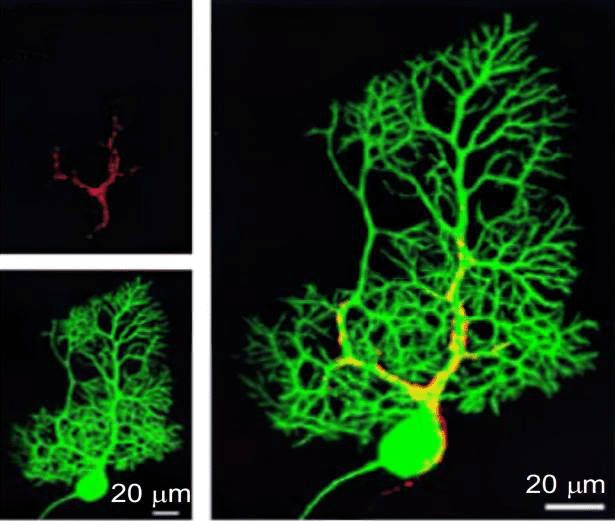
Figure.2 Calcium imaging at the synapse in vitro [11]
Application: Luciferase Report System in Neuron
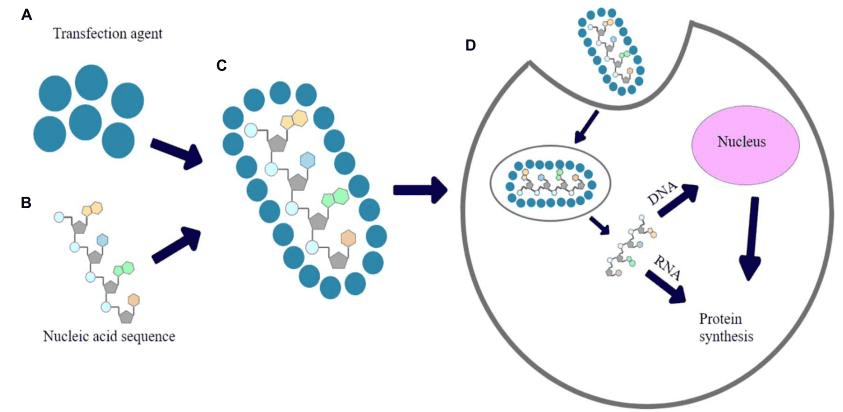
Luciferase assay can be used to investigate whether the protein can activate or repress the expression of the target gene[12]. The DNA structure with the gene promoter and the luciferase reporter gene coding region enter the cell through transfection. Another DNA structure introduced into the cell is composed of the coding region of the target protein. When this protein activates transcription, cells produce luciferase. Photometric quantification of luciferase activity is performed after adding lysis buffer and substrate.
Typically, in addition to one reporter gene as a detector, another reporter gene needs to be added as an internal reference, thus constituting a dual-luciferase reporter system. In the measurement of gene expression, dual reporters are generally used for transient transfections of cultured cells, where one vector containing the experimental reporter gene is co-transfected with another vector containing a distinct reporter gene as the control[13].
Reporters are coupled with regulatory promoters to investigate the structural or physiological basis of regulated gene expression. Because dual-luciferase reporter systems can measure cell signals in real-time and precisely, they are widely used in in vitro and in vivo experiments of neural cells, such as neuronal differentiation, neurogenesis, neuroinflammation, and brain injury.
In a publication published in 2005, Fukuda et al. constructed a chimeric gene encoding a luciferase reporter sequence upstream of the Notch1 3’UTR using the psiCheck2 vector to test the possibility that miRNAs regulate the Notch1 gene, a generally accepted regulator of neuronal differentiation[14]. In addition, Li Wenlu et al. constructed Hes6 and Atoh8 luciferase-reporter systems composed of two target genes of Ascl1 and verified that brain endothelial cells can release microvesicles to transfer pre-neural transcription factor Ascl1 to astrocytes, thus enabling them to complete neural differentiation[15]. Yun-Hee et al. used pRL-TK release to detect LRRK2 in epithelial neural cells damaged in vitro in a real-time assay for brain injury repair, verifying that LRRK2 can be specific and that the brain permeability inhibitor G1023 can significantly prevent brain tissue injury, cell death, and inflammatory response, and reduce motor and cognitive deficits caused by controlled cortical impact injury[16].
Dual-luciferase reporter systems can reduce the influence of internal variable factors on the accuracy of the experiment and make the experimental results not affected by the change of experimental conditions.
Mouse Primary Cortical Neurons Culture
Primary cultured neurons are often used in neural experiments, and we collated the culture methods of primary neural cells, which are suitable for the culture of mouse primary cortical neurons.
1. Primary cortical neurons were prepared from embryonic day 15.5 ICR mice with minor modifications.
2. Cortices were dissected in HBSS, followed by digestion in papain (20 U/ml) and DNase (10 U/ml) diluted in HBSS for 20 min at 37°C.
3. Enzyme-digested cortices were washed three times withDulbecco’s Modified Dagle Medium (DMEM/F12, 1% antibiotic-Anti Fungicin, 1% glutamine), 10% FBS, 10% heat-inactivated fetal bovine serum (FBS) and dissociated in culture medium.
4. For immunocytochemistry, cells were plated into 12-well culture plates at a density of 2 × 105cells per well with 18-mm glass covers coated with 50 μg/ml poly-D-lysine.
5. For RT-PCR and western blotting, isolated neural cells were plated into 6-well culture plates at a density of 8 × 105cells per well, respectively.
Conclusion
Due to its extremely sensitive, high signal background ratio, unparalleled dynamic range and versatility, the luciferase reporter system has been widely used in the study of nerve cell signaling regulatory mechanism. Luciferase helps scientists reduce the difficulty of studying neuronal differentiation, neurogenesis, neuroinflammation, brain injury and other aspects. It is an important research method in modern neural research.
Where to Get Luciferase Reporter Cell Line and Nervous System Primary Cells for Your Research?
AcceGen offers reporter stable cell lines for almost any application regardless of cell type with lentiviral-based transduction. AcceGen also offers a wide range of high-quality human neurons and glial cells, such as astrocytes, microglial cells, oligodendrocytes, and Schwann cells. These cell products provide you with a convenient means to research. To get more information, please refer to: Reporter Stable Cell Lines and Nervous System Primary Cells.
It is our pleasure to help relative researches to move forward. All the products of AcceGen are strictly comply with international standards. For more detailed information, please visit our product portfolio or contact [email protected].
References
1. Eun, HyoneMyong. Marker/Reporter Enzymes – Enzymology Primer for Recombinant DNA Technology – 8[J]. Enzymology Primer for Recombinant Dna Technology, 1996, 9(6):567–645.
2. Nakajima Y , Ohmiya Y . Bioluminescence assays: multicolor luciferase assay, secreted luciferase assay and imaging luciferase assay[J]. Expert Opin Drug Discov, 2010, 5(9):835-849.
3. Kimura A , Kobayashi E . Imaging Studies Using Reporter-Gene Transgenic Rats[J]. Encyclopedia of Neuroscience, 2009:97-102.
4. Keyaerts M, Caveliers V, Lahoutte T. Comprehensive Biomedical physics: Bioluminescence Imaging[M]//Comprehensive Biomedical physics: Bioluminescence Imaging. Elsevier, 2014: 245-256.
5. Eun, HyoneMyong. Marker/Reporter Enzymes – Enzymology Primer for Recombinant DNA Technology – 8[J]. Enzymology Primer for Recombinant Dna Technology, 1996, 9(6):567–645.
6. Berglund K , Clissold K , Li H E , et al. Luminopsins integrate opto- and chemogenetics by using physical and biological light sources for opsin activation[J]. Proceedings of the National Academy of Sciences, 2016.
7. Lagercrantz H . The Emergence of Consciousness in the Newborn[J]. Elsevier B.V. 2007.
8. Daroff R B, Aminoff M J. Encyclopedia of the neurological sciences[M]. Academic press, 2014.
9. Wise S P , Shadmehr R . Encyclopedia of the Human Brain[J]. Academic Press, 2002.
10. Wang H F , Wang G Y , Liu B , et al. Effect of glial cells on remyelination after spinal cord injury[J]. Neural Regeneration Research, 2017, 012(010):1724-1732.
11. Grienberger C , Konnerth A . Imaging calcium in neurons.[J]. Neuron, 2012, 73(5):862-885.
12. Carter M , Shieh J . Chapter 15. Biochemical Assays and Intracellular Signaling[M]. Elsevier Inc. 2015.
13. Sherf B A , Navarro S L , Hannah R R , et al. Dual-Luciferase TM Reporter Assay: An Advanced Co-Reporter Technology Integrating Firefly and Renilla Luciferase Assays[J]. Promega Note, 1996.
14. Fukuda Y, Kawasaki H, Taira K. Exploration of human miRNA target genes in neuronal differentiation[C]//Nucleic acids symposium series. Oxford University Press, 2005, 49(1): 341-342.
15. Li W, Mandeville E T, Durán-Laforet V, et al. Endothelial cells regulate astrocyte to neural progenitor cell trans-differentiation in a mouse model of stroke[J]. Nature Communications, 2022, 13(1): 7812.
16. Bae Y H, Joo H, Bae J, et al. Brain injury induces HIF-1α-dependent transcriptional activation of LRRK2 that exacerbates brain damage[J]. Cell death & disease, 2018, 9(11): 1125.

Copyright - Unless otherwise stated all contents of this website are AcceGen™ All Rights Reserved – Full details of the use of materials on this site please refer to AcceGen Editorial Policy – Guest Posts are welcome, by submitting a guest post to AcceGen you are agree to the AcceGen Guest Post Agreement – Any concerns please contact [email protected]