- In-Stock Tumor Cell Lines
- Human Orbital Fibroblasts
- Human Microglia
- Human Pulmonary Alveolar Epithelial Cells
- Human Colonic Fibroblasts
- Human Type II Alveolar Epithelial Cells
- Human Valvular Interstitial Cells
- Human Thyroid Epithelial Cells
- C57BL/6 Mouse Dermal Fibroblasts
- Human Alveolar Macrophages
- Human Dermal Fibroblasts, Adult
- Human Lung Fibroblasts, Adult
- Human Retinal Muller Cells
- Human Articular Chondrocytes
- Human Retinal Pigment Epithelial Cells
- Human Pancreatic Islets of Langerhans Cells
- Human Kidney Podocyte Cells
- Human Renal Proximal Tubule Cells
Introduction: CRISPR/Cas9 System and iPSCs
A reliable in vitro model is essential for physiology and disease research. The combination of CRISPR gene editing technology and iPSCs can provide researchers with highly customizable in vitro models (such as cell models or organoid models) with a purely genetic background, which provides a valuable tool for the researchers.
CRISPR/Cas9 System
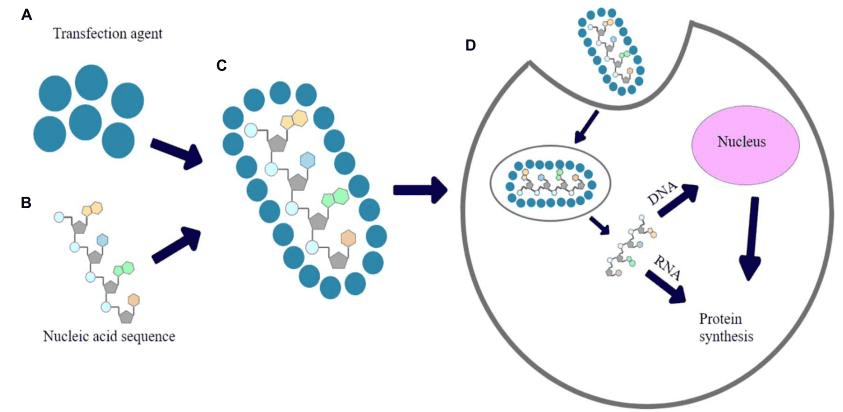
CRISPR/Cas9 system (clustered regularly interspaced short palindromic repeats/CRISPR-associated protein 9) (Figure.1) is the base of new generation gene edit technology. This technology greatly simplifies the operation steps and difficulties of gene editing, improves the efficiency of gene editing, and reduces the cost. CRISPR/Cas system is a prokaryotic immune mechanism that can resist foreign genetic elements. CRISPR refers to clustered regularly interspaced short palindromic repeats, which is a family of DNA sequences found in the genomes of prokaryotic organisms. Cas refers to CRISPR-associated protein, which can cleave specific strands of DNA under the guidance of sgRNA (single guide RNA)[1]. CRISPR/Cas9 system was first developed as a gene edit tool in eukaryotic cells by groups led by Zhang Feng and George Church in 2013[2; 3; 4]. Since then, this new generation of gene editing technology has achieved rapid development and wide application. But the application of the CRISPR/Cas9 system in clinical research and treatment has been accompanied by controversy. Except for the ethical issues, the inherent defects of the system (mistargeting due to lack of specificity and stability) may lead to unpredictable security risks.

Figure.1 CRISPR/Cas9 system[4]
Induced Pluripotent Stem Cells (iPSCs)
Induced pluripotent stem cells (also known as iPS cells or iPSCs, Figure.2) are a type of pluripotent stem cells that can be generated directly from somatic cells. iPSCs were established and published by Shinya Yamanaka’s team in 2006, and Shinya Yamanaka was awarded the 2012 Nobel Prize in Physiology or Medicine with John Gurdon[5; 6]. Yamanaka and his team tried to make somatic cells obtain the pluripotency of their differentiation precursors through gene editing, and finally they found that four specific factors (expressed by four genes: c-Myc, Oct3/4, Sox2 and Klf4) can convert somatic cells into pluripotent stem cells[5]. Pluripotent stem cells have great research significance and potential application value in the field of tissue regeneration. However, as the best choice for pluripotent cells, the research and application of embryonic stem cells were inevitably accompanied by huge ethical disputes before the invention of iPSCs[7; 8]. Therefore, the invention of iPSCs has great scientific significance.

Figure.2 Human iPSCs colonies culture on mouse fibroblast cells background.[9]
Advantages and Methods : CRISPR/Cas9 and iPSCs
Advantages
The application of the CRISPR/Cas9 system in iPSCs provides powerful assistance in various scientific and technological research in multiple fields. The research models based on CRISPR/Cas9-system-editing-iPSCs show unique advantages compared with immortalized cell lines and animal models in studying functional genomics, physiology, and pathology. iPSCs can be induced to differentiate into different somatic cells with high epigenetic consistency, which avoids the interference of possible uncontrollable variables caused by repeated CRISPR/Cas9 gene editing on other animal models or cell lines.
Methods
The principle and operation methods of CRISPR/Cas9 gene editing are easy and convenient. First, designing the sgRNA that guides the smooth completion of fixed-point cutting of Cas protein. The sgRNA-related websites are very valuable tools[10] during the design, and designing multiple sgRNAs to reduce the mistargeting rate is a more recommended strategy. Then we need to construct a transfection vector (plasmid) for sgRNA and Cas9 expression system. Multiple methods of transfection are available, such as liposome transfection procedures, viral transfection, and electroporation transfection[11]. At last, it is necessary to confirm whether the sequence editing is as expected through PCR and sequencing.
About the culture methods of iPSCs, here is a brief introduction to the culture protocol. In the cultivation system with trophoblast, the culture medium is mainly DMEM/F12 with fibroblast growth factor (bFGF) and serum (or non-serum substitution). The trophoblast cells mainly refer to cell cycle arrested mouse embryonic fibroblasts (MEF), which can provide growth factors, cytokine, and nutrients to iPSCs[12]. Besides, the cultivation systems of iPSCs were also iterated in recent years. The cultivation system without trophoblasts and animal-derived components (such as Essential 8 medium, E8) makes the culture of iPSCs easier than before[13].
Application of iPSCs-CRISPR/Cas9 in the Publication
Hemophilia B (HB) is a genetic bleeding disorder caused by a mutation in the coagulation factor 9 (F9) gene (X-linked recessive trait, the incidence rate is about 0.0002%~0.00025%) and characterized by a reduced or absent functional clotting factor IX (FIX) that is synthesized in the liver by the hepatocytes. Luce et al. tried to discover the possibility of recovering the FIX activity through iPSCs-CRISPR/Cas9 to rescue the bleeding phenotype of HB[14]. They have developed HB hepatocytes in vitro model through iPSCs generated from fibroblasts of a severe HB patient and tried to enhance FIX activity through CRISPR/Cas9 system to target the genomic insertion of a coagulation factor 9 minigene with the Padua mutation. After 2D and 3D induced differentiation procedure, iPSCs with (corr-iPSCs) or without (HB-iPSCs) FIX activity correction was differentiated into immature (2D) and mature (3D) Hepatocytes (corr-iHeps and HB-iHeps). And then, they transplanted these two mature types of iHeps into the liver of the F9 KO mouse (HB mouse model). They found that the correction of FIX activity can only succeed in mature iHeps, and corr-iHeps transplantation can successfully rescue of bleeding phenotype in the HB mouse model. These results illustrate the great potential of iPSCs combined with CRISPR/Cas9 gene editing technology in the treatment of hemophilia B.
Conclusion
CRISPR/Cas9 combined with iPSCs shows the high value and broad prospects in the research of various fields, such as gene therapy, genetic function identification, organ regeneration, drug experiment, etc. Therefore, this field is worth undertaking and making continuous efforts for researchers.
Where to Get Knockout Stable Cell Lines, iPSCs for Your Research?
AcceGen offers a series of gene knockout cell lines developed by the CRISPR/Cas9 system and a wide range of high-quality human/animal stem cells, including iPSCs, MSCs, ESCs, Adult Stem Cells, and Tumor Stem Cells. These cell lines provide you with a convenient means to research. To get more information, please refer to: Knockout Stable Cell Lines or Stem Cells.
It is our pleasure to help relative researches to move forward. All the products of AcceGen are strictly comply with international standards. For more detailed information, please visit our product portfolio or contact [email protected].
References
[1] R. Barrangou, The roles of CRISPR-Cas systems in adaptive immunity and beyond. Curr Opin Immunol 32 (2015) 36-41.
[2] P. Mali, L. Yang, K.M. Esvelt, J. Aach, M. Guell, J.E. DiCarlo, J.E. Norville, and G.M. Church, RNA-guided human genome engineering via Cas9. Science 339 (2013) 823-6.
[3] L. Cong, F.A. Ran, D. Cox, S. Lin, R. Barretto, N. Habib, P.D. Hsu, X. Wu, W. Jiang, L.A. Marraffini, and F. Zhang, Multiplex genome engineering using CRISPR/Cas systems. Science 339 (2013) 819-23.
[4] P.D. Hsu, E.S. Lander, and F. Zhang, Development and applications of CRISPR-Cas9 for genome engineering. Cell 157 (2014) 1262-1278.
[5] K. Takahashi, and S. Yamanaka, Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126 (2006) 663-76.
[6] https://www.nobelprize.org/nobel_prizes/medicine/laureates/2012/press.html, The Nobel Prize in Physiology or Medicine – 2012 Press Release. Nobel Media AB.
[7] R.S. Mahla, Stem Cells Applications in Regenerative Medicine and Disease Therapeutics. Int J Cell Biol 2016 (2016) 6940283.
[8] I. Klimanskaya, Y. Chung, S. Becker, S.J. Lu, and R. Lanza, Human embryonic stem cell lines derived from single blastomeres. Nature 444 (2006) 481-5.
[9] wikipedia, https://encyclopedia.thefreedictionary.com/Induced+pluripotent+stem+cell.
[10] crispr.mit.edu, https://zlab.bio/guide-design-resources.
[11] B.C. Geng, K.H. Choi, S.Z. Wang, P. Chen, X.D. Pan, N.G. Dong, J.K. Ko, and H. Zhu, A simple, quick, and efficient CRISPR/Cas9 genome editing method for human induced pluripotent stem cells. Acta Pharmacol Sin 41 (2020) 1427-1432.
[12] J.C. Chiang, J. Jiang, P.E. Newburger, and J.B. Lawrence, Trisomy silencing by XIST normalizes Down syndrome cell pathogenesis demonstrated for hematopoietic defects in vitro. Nat Commun 9 (2018) 5180.
[13] G. Chen, D.R. Gulbranson, Z. Hou, J.M. Bolin, V. Ruotti, M.D. Probasco, K. Smuga-Otto, S.E. Howden, N.R. Diol, N.E. Propson, R. Wagner, G.O. Lee, J. Antosiewicz-Bourget, J.M. Teng, and J.A. Thomson, Chemically defined conditions for human iPSC derivation and culture. Nat Methods 8 (2011) 424-9.
[14] E. Luce, C. Steichen, M. Allouche, A. Messina, J.M. Heslan, T. Lambert, A. Weber, T.H. Nguyen, O. Christophe, and A. Dubart-Kupperschmitt, In vitro recovery of FIX clotting activity as a marker of highly functional hepatocytes in a hemophilia B iPSC model. Hepatology 75 (2022) 866-880.

Copyright - Unless otherwise stated all contents of this website are AcceGen™ All Rights Reserved – Full details of the use of materials on this site please refer to AcceGen Editorial Policy – Guest Posts are welcome, by submitting a guest post to AcceGen you are agree to the AcceGen Guest Post Agreement – Any concerns please contact [email protected]