- In-Stock Tumor Cell Lines
- Human Orbital Fibroblasts
- Human Microglia
- Human Pulmonary Alveolar Epithelial Cells
- Human Colonic Fibroblasts
- Human Type II Alveolar Epithelial Cells
- Human Valvular Interstitial Cells
- Human Thyroid Epithelial Cells
- C57BL/6 Mouse Dermal Fibroblasts
- Human Alveolar Macrophages
- Human Dermal Fibroblasts, Adult
- Human Lung Fibroblasts, Adult
- Human Retinal Muller Cells
- Human Articular Chondrocytes
- Human Retinal Pigment Epithelial Cells
- Human Pancreatic Islets of Langerhans Cells
- Human Kidney Podocyte Cells
- Human Renal Proximal Tubule Cells
Lung diseases, especially lung inflammation, are one of the most concerning diseases in the SARS-Cov-2 pandemic era. As the core immune cells that play key roles in the SARS-Cov-2 viral pneumonia, alveolar macrophages have become a key research object for the research of potential clinical treatment methods and targets.
Lung and Alveoli
Lung
The lung is the primary organ of the respiration system and a part of the lower respiratory tract. The function of the lung is gas exchange between the atmosphere and the bloodstream. The oxygen from the air is extracted and transferred to the bloodstream, and the carbon dioxide is released from the bloodstream to the atmosphere[1]. The left and right lungs are located inside the chest cavity on both sides of the heart, and the function area is the respiratory zone that connects the bronchus end and mainly consists of the alveoli[2, 3]. Besides the main function area, the main components of the lung also include the airway and its epithelial system with gas transport and neuromodulation that serve respiration, the connective tissue that provides the mechanical expansion and structural support for the lung, and the functionally complex microbiota[4-6]. The most common lung diseases are inflammation and infection, which are also often the precursor to more serious lung diseases such as cancer, restrictive and obstructive lung diseases. Besides, vascular-embolism-induced inadequate blood supply to the lungs and some congenital diseases are also diseases that affect the physiological function of the lungs[7, 8].
Alveoli
The alveoli as the functional unit of the lung are the main place where gas exchange processes take place for mammals, which is a large number of expandable cup-like lumens distributed around the fine bronchi[9]. (Figure.1) And the alveolar cells mainly include type Ⅰ, type Ⅱ, and alveolar macrophages. Type Ⅰ alveolar cells are thin, flattened squamous cells without self-replicating ability, which form the structure of alveoli. As the main component of the alveolar surface, type I alveolar cells form an air-blood barrier with the basement membrane, the thin layer of connective tissue, capillary basement membrane, and endothelium. Type II alveolar cells are primarily responsible for releasing surfactants to reduce respiratory membrane surface tension and retain some differentiation potential to replace damaged type I cells. Type 2 cells are distributed between type I alveolar cells, with a greater number than type I alveolar cells, but a smaller coverage area than type I alveolar cells. The cells are cubic or circular, with their tips protruding into the alveolar cavity[10]. And alveolar macrophages come from blood monocytes, which reside on the internal luminal surfaces of the alveoli, the alveolar ducts, and the bronchioles. It can remove foreign bodies (such as dust, pathogens, carbon particles, and blood cells from injuries) from the alveolar structures to ensure the alveoli are working continuously in a normal physiological environment[11].
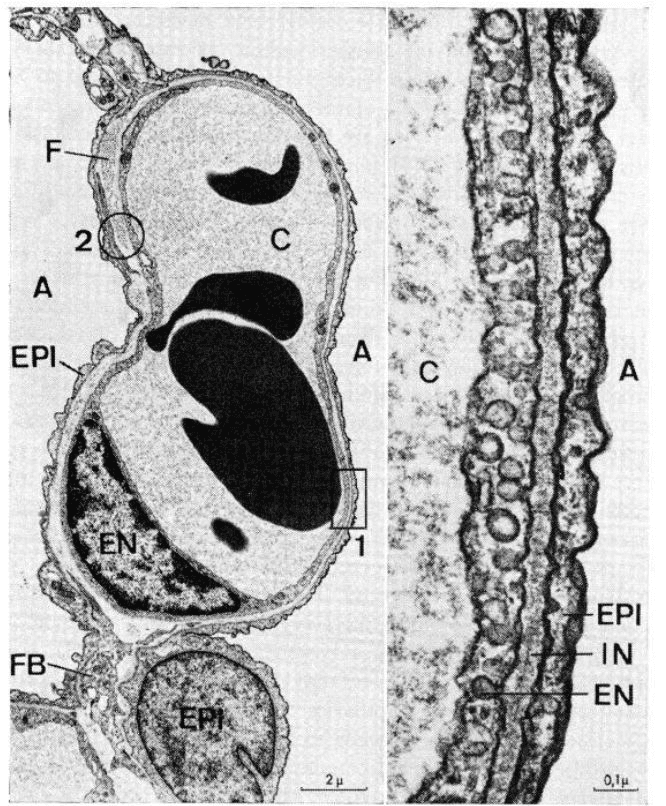
Figure.1 alveoli (left) and air-blood barrier in alveoli(right).[12]
Alveolar Macrophages and Human-Derived In Vitro Models
Alveolar macrophages are high-activity macrophages that can be identified as professional devourers, which are developed from phagocytes migrating to the lung and belonging to the mononuclear phagocyte system. It plays key roles in maintaining the in vivo balance, host defense, and organizational reinvention[11, 13]. Proper execution of phagocytosis by alveolar macrophages depends on multiple signaling regulatory mechanisms, such as nitric oxide (NO) signal, prostaglandin endoperoxide 2 (PGE2), Interleukin signal (mainly IL-4 and IL-10), transforming growth factor β (TGF-β), and Toll-like receptors (TLR) signal[14-16]. Therefore, pathological mechanisms of alveolar macrophages show great value for exploring potential treatment methods for lung diseases, especially in the context of the current SARS-Cov-2 global pandemic.
Primary cells are ideal in vitro models for physiological and pathological research, which can provide a pure genetic background and thus closer to the actual physiological environment in vivo. In the research of lung diseases, the application of in vitro models based on primary cells is commonly found in high-impact publications, of which primary alveolar macrophages are one. Besides, alveolar macrophage-like cells (AM-like cells) can be differentiated from mouse bone marrow cells and fetal liver cells, which is another source of AM-like in vitro models for the research. Hence, we will give a brief introduction to the isolation and culture methods protocol[17].
The source of primary human alveolar macrophages is clinical bronchoalveolar lavage (BAL) fluid samples. The samples are transferred to the laboratory under 4℃ and centrifuged to an isolated cell pellet from BAL. Wash the cell pellet with PBS containing heat-inactivated FBS and EDTA, then centrifuge. After resuspending the cells in a flow/sorting mixture (PBS, Heat inactivated FBS, EDTA, HEPES), use human Fc block and then incubate on ice to block the nonspecific antibody binding to the Fc receptor. And then, the alveolar macrophages are isolated from BAL cells through antibody staining and single-cell flow cytometry sorting. The primary human alveolar macrophages need to culture in the RPMI 1640 with FBS, M-CSF, sodium pyruvate, HEPES, and penicillin/streptomycin, and the culture environment is 37℃ with 5% CO2.
Application of Primary Alveolar Macrophages in the research of SARS-Cov-2
The SARS-Cov-2 global pandemic causes serious public health problems, viral pneumonia has caused many severe illnesses and deaths worldwide, and the main cause is the severe inflammation and immune response. Of course, alveolar macrophages as high-activity immune cells in the lung, which play essential roles in the pathology of viral pneumonia. And primary alveolar macrophages as an in vitro model show great value. Hoepel, et al. use human primary alveolar macrophages as in vitro models to dissect a mechanism for the severe inflammatory immune response in severe SARS-Cov-2 viral pneumonia cases[18]. They found that the excessive immune response of human alveolar macrophages in critically ill patients is induced by SARS-CoV-2 spike protein-specific IgG. The excessive immune response is mainly caused by high titer and aberrant glycosylation of anti-spike IgG. And then, the researchers identified the Fcγ receptors FcγRIIa and FcγRIII are the main targets of anti-spike IgG and successfully counteracted the inflammation induced by anti-spike IgG through blocking the target receptors by an FDA- and EMA-approved therapeutic small molecule.
Conclusion
Lung disease has been one of the most concerning fields in the past few years of the SARS-Cov-2 pandemic, and the number of SARS-Cov-2-relates publications are high up to nearly 200 thousand in the past 3 years (Figure.2). Alveolar Macrophages play key roles in the pathological mechanisms of inflammation and immune of viral pneumonia, which are immune cells that are difficult to evade in related research. Therefore, mastering the powerful in vitro model of human primary alveolar macrophages is of great value to researchers.

Figure.2 Number of publications relate to SARS-Cov-2.[19]
Where to Get Respiratory System Primary Cells for Your Research?
AcceGen isolates and offers a wide range of high-quality respiratory system primary cells, such as Human Alveolar Macrophages, Human Type II Alveolar Epithelial Cells, Human Pulmonary Alveolar Epithelial Cells, and Human Pulmonary Pericytes. These cell products provide you with a convenient means to research. To get more information, please refer to: Respiratory System Primary Cells.
It is our pleasure to help relative researches to move forward. All the products of AcceGen are strictly comply with international standards. For more detailed information, please visit our product portfolio or contact [email protected].
References
[1] Tortora GJ, Anagnostakos NP. Principles of Anatomy and Physiology: Harper & Row, 1987.
[2] Drake R, Vogl AW, Mitchell AWM. Gray’s Anatomy for Students E-Book: Elsevier Health Sciences, 2014.
[3] Standring S, Borley NR. Gray’s Anatomy: The Anatomical Basis of Clinical Practice: Churchill Livingstone/Elsevier, 2008.
[4] Lommel AV. Pulmonary neuroendocrine cells (PNEC) and neuroepithelial bodies (NEB): chemoreceptors and regulators of lung development. Paediatric Respiratory Reviews 2001;2:171-176.
[5] Weinberger SE, Cockrill BA, Mandel J. Principles of Pulmonary Medicine: Elsevier, 2018.
[6] Hiemstra PS, McCray PB, Jr., Bals R. The innate immune function of airway epithelial cells in inflammatory lung disease. Eur Respir J 2015;45:1150-1162.
[7] Walker BR, Colledge NR, Ralston SH, Penman I. Davidson’s Principles and Practice of Medicine: Churchill Livingstone/Elsevier, 2014.
[8] Sieunarine K, May J, WHITE GH, Harris JP. ANOMALOUS AZYGOS VEIN: A POTENTIAL DANGER DURING ENDOSCOPIC THORACIC SYMPATHECTOMY. Australian and New Zealand Journal of Surgery 1997;67:578-579.
[9] Knudsen L, Ochs M. The micromechanics of lung alveoli: structure and function of surfactant and tissue components. Histochem Cell Biol 2018;150:661-676.
[10] Koeppen BM, Stanton BA. Berne and Levy Principles of Physiology: Mosby, 2008.
[11] Lambrecht BN. Alveolar macrophage in the driver’s seat. Immunity 2006;24:366-368.
[12] Weibel ER. Morphological basis of alveolar-capillary gas exchange. Physiol Rev 1973;53:419-495.
[13] Weinberger SE, Cockrill BA, Mandel J. Principles of Pulmonary Medicine: Elsevier, 2019.
[14] Bingisser RM, Holt PG. Immunomodulating mechanisms in the lower respiratory tract: nitric oxide mediated interactions between alveolar macrophages, epithelial cells, and T-cells. Swiss Med Wkly 2001;131:171-179.
[15] Krutzik SR, Modlin RL. The role of Toll-like receptors in combating mycobacteria. Semin Immunol 2004;16:35-41.
[16] Pouliot P, Turmel V, Gélinas E, Laviolette M, Bissonnette EY. Interleukin-4 production by human alveolar macrophages. Clin Exp Allergy 2005;35:804-810.
[17] Nayak DK, Mendez O, Bowen S, Mohanakumar T. Isolation and In Vitro Culture of Murine and Human Alveolar Macrophages. J Vis Exp 2018.
[18] Hoepel W, Chen HJ, Geyer CE, Allahverdiyeva S, Manz XD, de Taeye SW, Aman J, et al. High titers and low fucosylation of early human anti-SARS-CoV-2 IgG promote inflammation by alveolar macrophages. Sci Transl Med 2021;13.
[19] NCBI-Pubmed. https://pubmed.ncbi.nlm.nih.gov/?term=sars%20cov2&sort=date&size=200&timeline=expanded.

Copyright - Unless otherwise stated all contents of this website are AcceGen™ All Rights Reserved – Full details of the use of materials on this site please refer to AcceGen Editorial Policy – Guest Posts are welcome, by submitting a guest post to AcceGen you are agree to the AcceGen Guest Post Agreement – Any concerns please contact [email protected]