- In-Stock Tumor Cell Lines
- Human Orbital Fibroblasts
- Human Microglia
- Human Pulmonary Alveolar Epithelial Cells
- Human Colonic Fibroblasts
- Human Type II Alveolar Epithelial Cells
- Human Valvular Interstitial Cells
- Human Thyroid Epithelial Cells
- C57BL/6 Mouse Dermal Fibroblasts
- Human Alveolar Macrophages
- Human Dermal Fibroblasts, Adult
- Human Lung Fibroblasts, Adult
- Human Retinal Muller Cells
- Human Articular Chondrocytes
- Human Retinal Pigment Epithelial Cells
- Human Pancreatic Islets of Langerhans Cells
- Human Kidney Podocyte Cells
- Human Renal Proximal Tubule Cells
Adrenal Cortex and Diseases
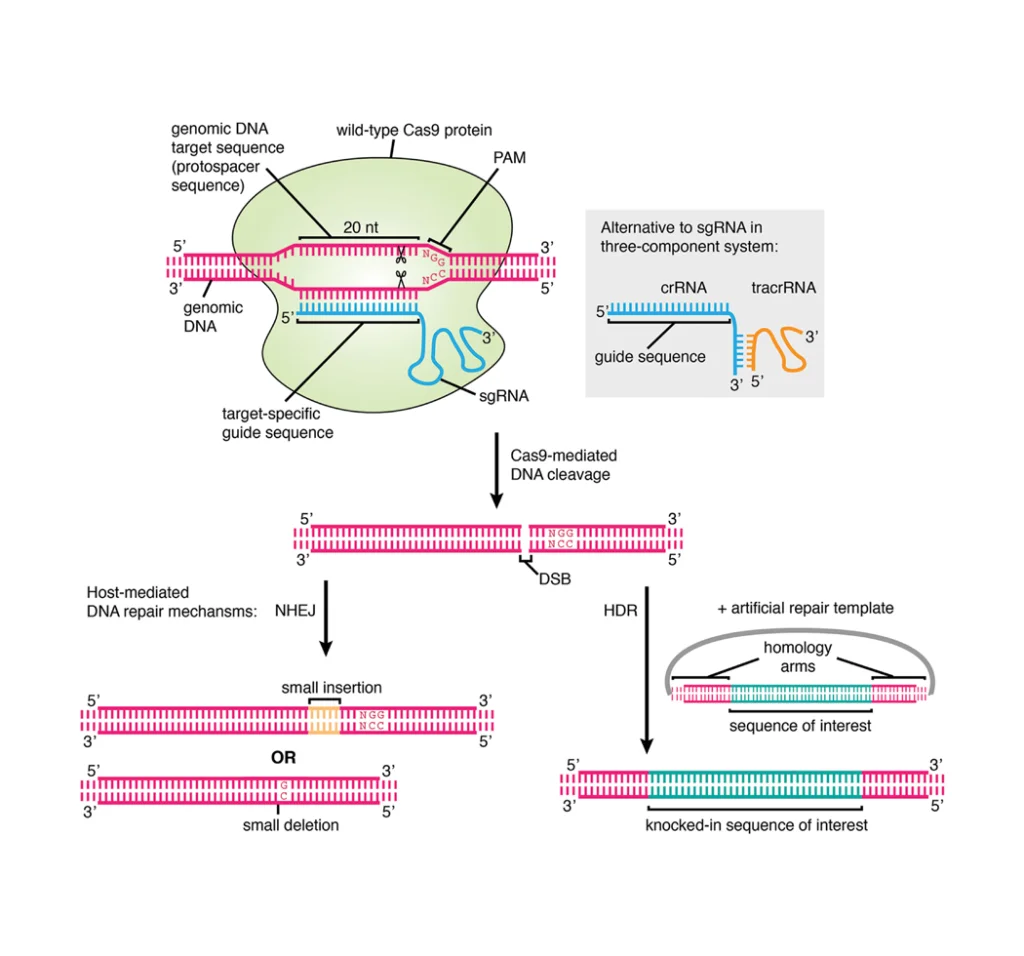
The adrenal cortex is the largest part and the outer region of the adrenal gland, which is a portion of hormone secretion sites. Adrenal Cortex is the main place for the synthesis of various corticosteroid hormones, such as mineralocorticoids, glucocorticoids and androgens[1; 2]. Zona glomerulosa (ZG), zona fasciculata (ZF) and zona reticularis (ZR) are three main zones of the adrenal cortex separately[3]. (Figure.1a&b) ZG is the main place for the production and secretion of mineralocorticoid aldosterone, which is regulated by renin–angiotensin–aldosterone axis and adrenocorticotropic hormone (ACTH)[4; 5]. However, ZG cannot synthesize cortisol, corticosterone or sex hormones due to the absence of the expression of 11β-hydroxylase and 17α-hydroxylase[2]. ZF is the main site for the production of glucocorticoids, as well as small amounts of adrenal androgens and estrogens[6]. The secretion of glucocorticoids is also regulated by ACTH[5]. ZR is the inner layer of the adrenal cortex, which mainly produces adrenal androgens and small amounts of estrogens and glucocorticoids[6].
(a)
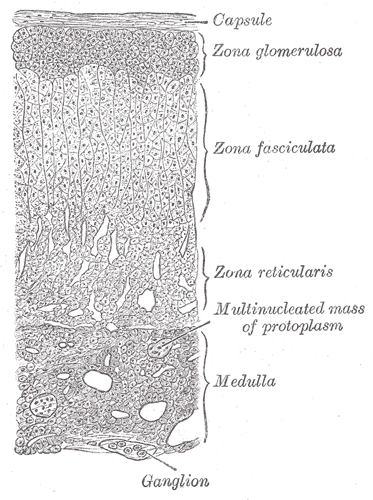
(b)

Figure.1 Structure of adrenal gland and adrenal cortex[7; 8]. (a) Schematic diagram of adrenal cortex structure. (b) Adrenal tissue section
Diseases related to the adrenal cortex mainly originate from abnormal hormone levels due to the abnormal function of the adrenal cortex. Adrenal insufficiency can lead to inadequate amounts of steroid hormones. The lack of these hormones can seriously affect blood pressure, electrolytes, and metabolic balance[9; 10]. Adrenal cortex insufficiency can be divided into primary and secondary. Primary adrenal cortex insufficiency refers to the destruction (such as Addison’s disease), developmental failure, or enzyme deficiency of the adrenal gland itself[10]. Secondary adrenal cortex insufficiency refers to the adrenal cortex insufficiency caused by the inability of the upstream of the adrenal gland (pituitary gland and hypothalamus) to produce enough hormones (such as ACTH and CRH) to promote adrenal function[11]. By contrast, excessive hormone levels are another aspect of Adrenal cortex disease. Primary (such as primary aldosteronism, PA) and secondary (such as Cushing’s syndrome or Cushing’s disease) excessive hormones from the adrenal cortex can also lead to serious consequences[12; 13]. Besides, malignant tumors can also happen in the adrenal cortex. Adrenocortical carcinoma (ACC) is aggressive cancer originating in the cortex (steroid hormone-producing tissue) of the adrenal gland, which mainly causes the oversecretion of hormones in the adrenal cortex[14].
Human Adrenal Cortical Cells and Culture Protocol
Adrenal cortical cell lines and primary cells are both useful tools in laboratory in vitro research. SW13 (human adrenal cortex adenocarcinoma cell line) and Y-1 (mouse adrenal cortex cell line), are the most widely used in vitro immortalized models[15, 16]. However, primary cells, especially human source primary cells, are indispensable and reliable in vitro models in high-level publications. Human primary adrenal cortical cells were successfully isolated from fetal adrenal and cultured in vitro firstly, and the isolation and in vitro culture methods of adult normal adrenal cortical cells were delayed for some years due to various difficulties. The adrenal cortex of newborns is similar to that of adults with only ZR layers, while adults exhibit a complete three-layer structure (ZG, ZF, ZR) of the adrenal cortex, which is one of the important reasons for the relatively difficult separation of adult adrenal cortex cells. [17]. Selective separation of primary cultures according to a three-layer structure is unnecessary for most application scenarios, but if isolation is required, mechanical anatomy combined with antibody screening is used to isolate cells from specific layers of the adrenal cortex.
In this section, we give a brief introduction to the isolation and culture protocol of human normal adrenal cortical primary cells[17]. Fresh normal adrenal gland, adrenocortical carcinoma, or adrenal adenoma biopsy was collected and minced into small pieces. The tissue was digested in DMEM/F12 with 5000U/mL Pen/Strep, 50mg/mL Gentamicin, 0.1% collagenase, and 0.01% DNase І. After the digestion process, the tissue lysis was washed several times with DMEM/F12 and then cultured at 37℃, 5% CO2. In the first 4 days, the culture medium (DMEM/F12 with Pen/Strep, Gentamicin, and 10% Cosmic Calf Serum) needs the addition of 50mg/mL kanamycin.
Application of Human Cortical Cells
Human primary cortical cells are widely used in the research related to adrenal gland physiology and pathology. In a publication about the pathology of aldosterone-producing adenomas, Wu et al. discovered the mutation of cell adhesion molecule 1 (CADM1) plays a key role in adenomas-induced primary aldosteronism (PA) [18]. Briefly, they found the CADM1 mutation in the clinical samples from patients with PA and identified the pathological changes of CADM1 mutation in human adrenal cortical primary cells and cell lines. They observed that CADM1 mutation increased the expression of CYP11B2 and the production of aldosterone. Additionally, they identified the CADM1 mutation inhibits the gap junction intercellular communications (GJICs) to increase the expression of CYP11B2 and then lead to PA and PA-related hypertension. In another publication, Niemeyer et al. found that the infection of varicella-zoster virus (VZV) to primary human adrenal cortex cells leads to a proinflammatory environment without cell death[19]. They identified that VZV can effectively infect the cells without causing cell death, and its infection increases the expression of proinflammatory cytokines (such as ILs and TNF-α) in human primary adrenal cortical cells.
Conclusion
The adrenal cortex is one of the main hormone production and secretion organs, which relate to various metabolic pathways and diseases. Therefore, the adrenal cortex is a hot area in the research of physiology and medicine. As a useful and valuable tool, human adrenal cortical cells can be a reliable in vitro model for the researchers to complete their work.
Where to Get Endocrine System Primary Cells for Your Research?
AcceGen isolates and offers a wide range of high-quality endocrine system primary cells, such as Human Adrenal Cortical Cells, Human Adrenal Capsule Fibroblasts Cells, Human Pancreatic Islet Beta Cells, and Human Pancreatic Stromal Cells. These cell products provide you with a convenient means to research. To get more information, please refer to: Endocrine System Primary Cells.
It is our pleasure to help relative researches to move forward. All the products of AcceGen are strictly comply with international standards. For more detailed information, please visit our product portfolio or contact inquiry@accegen.com.
References
[1] K.M. Curnow, M.T. Tusie-Luna, L. Pascoe, R. Natarajan, J.L. Gu, J.L. Nadler, and P.C. White, The product of the CYP11B2 gene is required for aldosterone biosynthesis in the human adrenal cortex. Mol Endocrinol 5 (1991) 1513-22.
[2] J. Yuan, K.E. Barrett, S.M. Barman, and H.L. Brooks, Ganong’s Review of Medical Physiology, Twenty sixth Edition, McGraw-Hill Education, 2019.
[3] S.S. Nussey, and S.A. Whitehead, Endocrinology: An Integrated Approach, BIOS Scientific Publishers, 2001.
[4] J.H. Fountain, J. Kaur, and S.L. Lappin, Physiology, Renin Angiotensin System, StatPearls, StatPearls Publishing
Copyright © 2023, StatPearls Publishing LLC., Treasure Island (FL) ineligible companies. Disclosure: Jasleen Kaur declares no relevant financial relationships with ineligible companies. Disclosure: Sarah Lappin declares no relevant financial relationships with ineligible companies., 2023.
[5] A. Hanukoglu, D. Fried, I. Nakash, and I. Hanukoglu, Selective increases in adrenal steroidogenic capacity during acute respiratory disease in infants. Eur J Endocrinol 133 (1995) 552-6.
[6] J.E. Hall, and M.E. Hall, Guyton and Hall Textbook of Medical Physiology, Elsevier, 2020.
[7] https://en.wikipedia.org/wiki/File:Gray1185.png.
[8] https://en.wikipedia.org/wiki/File:Adrenal_cortex_labelled.jpg.
[9] K.I. Alexandraki, K. Sanpawithayakul, and A. Grossman, Adrenal Insufficiency. in: K.R. Feingold, B. Anawalt, M.R. Blackman, A. Boyce, G. Chrousos, E. Corpas, W.W. de Herder, K. Dhatariya, K. Dungan, J. Hofland, S. Kalra, G. Kaltsas, N. Kapoor, C. Koch, P. Kopp, M. Korbonits, C.S. Kovacs, W. Kuohung, B. Laferrère, M. Levy, E.A. McGee, R. McLachlan, M. New, J. Purnell, R. Sahay, A.S. Shah, F. Singer, M.A. Sperling, C.A. Stratakis, D.L. Trence, and D.P. Wilson, (Eds.), Endotext, MDText.com, Inc.
Copyright © 2000-2023, MDText.com, Inc., South Dartmouth (MA), 2000.
[10] M.R. Huecker, B.S. Bhutta, and E. Dominique, Adrenal Insufficiency, StatPearls, StatPearls Publishing
Copyright © 2023, StatPearls Publishing LLC., Treasure Island (FL) ineligible companies. Disclosure: Beenish Bhutta declares no relevant financial relationships with ineligible companies. Disclosure: Elvita Dominique declares no relevant financial relationships with ineligible companies., 2023.
[11] E. Brender, C. Lynm, and R.M. Glass, JAMA patient page. Adrenal insufficiency. Jama 294 (2005) 2528.
[12] C. Schirpenbach, and M. Reincke, Primary aldosteronism: current knowledge and controversies in Conn’s syndrome. Nat Clin Pract Endocrinol Metab 3 (2007) 220-7.
[13] G. AI, G. IP, Z. W, K. Z, and M. B, Pathology, Radom University, 2009.
[14] C. Wang, Y. Sun, H. Wu, D. Zhao, and J. Chen, Distinguishing adrenal cortical carcinomas and adenomas: a study of clinicopathological features and biomarkers. Histopathology 64 (2014) 567-76.
[15] V. Buonassisi, G. Sato, and A.I. Cohen, Hormone-producing cultures of adrenal and pituitary tumor origin. Proc Natl Acad Sci U S A 48 (1962) 1184-90.
[16] A. Leibovitz, W.M. McCombs, 3rd, D. Johnston, C.E. McCoy, and J.C. Stinson, New human cancer cell culture lines. I. SW-13, small-cell carcinoma of the adrenal cortex. J Natl Cancer Inst 51 (1973) 691-7.
[17] K. Nanba, A.R. Blinder, and W.E. Rainey, Primary Cultures and Cell Lines for In Vitro Modeling of the Human Adrenal Cortex. The Tohoku Journal of Experimental Medicine 253 (2021) 217-232.
[18] X. Wu, E.A.B. Azizan, E. Goodchild, S. Garg, M. Hagiyama, C.P. Cabrera, F.L. Fernandes-Rosa, S. Boulkroun, J.L. Kuan, Z. Tiang, A. David, M. Murakami, C.A. Mein, E. Wozniak, W. Zhao, A. Marker, F. Buss, R.S. Saleeb, J. Salsbury, Y. Tezuka, F. Satoh, K. Oki, A.M. Udager, D.L. Cohen, H. Wachtel, P.J. King, W.M. Drake, M. Gurnell, J. Ceral, A. Ryska, M. Mustangin, Y.P. Wong, G.C. Tan, M. Solar, M. Reincke, W.E. Rainey, R.S. Foo, Y. Takaoka, S.A. Murray, M.C. Zennaro, F. Beuschlein, A. Ito, and M.J. Brown, Somatic mutations of CADM1 in aldosterone-producing adenomas and gap junction-dependent regulation of aldosterone production. Nat Genet 55 (2023) 1009-1021.
[19] C.S. Niemeyer, T. Mescher, A.N. Bubak, E.M. Medina, J.E. Hassell, Jr., and M.A. Nagel, VZV Infection of Primary Human Adrenal Cortical Cells Produces a Proinflammatory Environment without Cell Death. Viruses 14 (2022).

Copyright - Unless otherwise stated all contents of this website are AcceGen™ All Rights Reserved – Full details of the use of materials on this site please refer to AcceGen Editorial Policy – Guest Posts are welcome, by submitting a guest post to AcceGen you are agree to the AcceGen Guest Post Agreement – Any concerns please contact marketing@accegen.com