- In-Stock Tumor Cell Lines
- Human Orbital Fibroblasts
- Human Microglia
- Human Pulmonary Alveolar Epithelial Cells
- Human Colonic Fibroblasts
- Human Type II Alveolar Epithelial Cells
- Human Valvular Interstitial Cells
- Human Thyroid Epithelial Cells
- C57BL/6 Mouse Dermal Fibroblasts
- Human Alveolar Macrophages
- Human Dermal Fibroblasts, Adult
- Human Lung Fibroblasts, Adult
- Human Retinal Muller Cells
- Human Articular Chondrocytes
- Human Retinal Pigment Epithelial Cells
- Human Pancreatic Islets of Langerhans Cells
- Human Kidney Podocyte Cells
- Human Renal Proximal Tubule Cells
What Is Transfection?
Transfection is the process of delivering nucleic acids into eukaryotic cells by nonviral methods, while minimizing unintended effects such as off-target gene perturbation, cytotoxicity, or cell death. Transfection is a fundamental technique in genetic manipulation, which allows scientists to intentionally introduce external genetic material into cells. This technique overcomes the inherent challenge of introducing negatively charged molecules – DNA or RNA of interest into cells with negatively charged plasma membranes . There are various methods can be used to facilitate this process, chemical reagents, cationic lipids, nonliposomal reagents, and physical methods including microinjection, electroporation, and biolistic particle delivery. Figure 1 shows the illustration of the mechanism of transfection [1]. Such introductions of foreign nucleic acid can result in a change of the properties of cells, allowing the study of gene function and protein expression in a cellular environment. By manipulating genetic material within cells, scientists are able to examine intricate cellular mechanisms, study various diseases, engineer genetically modified organisms, or produce therapeutic proteins for different medical purposes.
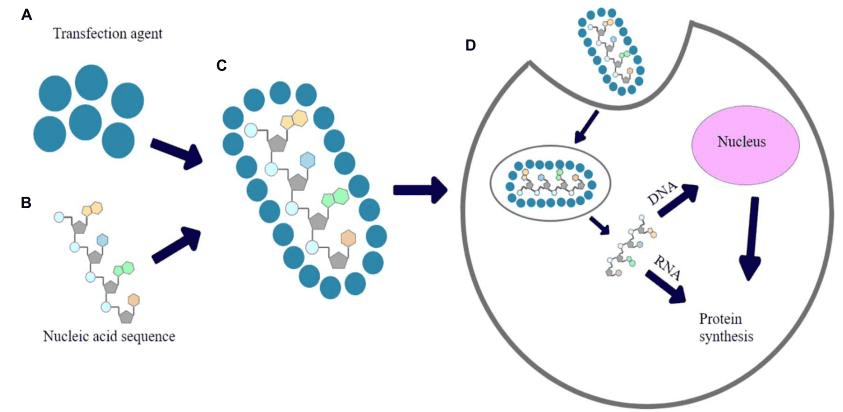
Figure 1. The proposed mechanism of transfection.
Types of Transfection
Although there are plenty of methods for introducing nucleic acids into cells, not all of these methods are applicable to all types of cells and experimental applications. There is a wide variation amongst them with respect to cell toxicity, transfection efficiency, effects on cellular physiology, and level of gene expression. However, based on how long the introduced nucleic acids exist in transfected cells, all of the transfection strategies can be further classified into two types: transient transfection and stable transfection.
If the introduced nucleic acid only expressed in the transfected cells for a limited period of time and does not replicate, such a transfection is transient transfection. Transient transfection only results in short-term gene expression. Otherwise, if the introduced nucleic acid is stable and typically integrated into the genome of the recipient, and persists in the transfected cells when the host genome replicates, then it is stable transfection. Stable transfection ensures consistent gene expression over time.
The Process of Stable Transfection
Stable transfection also begins with a transient transfection, it involves the intentional introduction of foreign genetic material typically through plasmids into host cells, followed by an infrequent but important and serendipitous process. Typically, in a small proportion of transfected cells, the foreign gene is integrated into the cells’ genome, and the transgene will then be constitutively expressed even with the replication of cells. Because stable integration of foreign gene into the genome is a relatively rare event, successful stable transfection requires both effective gene delivery and a way to select cells that have acquired the new gene. One of the most reliable ways to select cells that stably express the transfected DNA is to include a selectable marker in the DNA construct used for transfection. The most common way is to accomplish the selection by including an appropriate drug-resistance marker in the transfected DNA. Co-expressing the selectable marker with the gene of interest allows researchers to identify and select the cells that have the foreign DNA integrated into their genome while also selecting them against the transiently transfected cells.
For example, a commonly used selectable marker neo, which is the neomycin resistance gene can be co-transfect with the new gene for antibiotic resistance. Then the transiently transfected cells are treated with the appropriate antibiotic, in this case G418 (geneticin) for the selection of neo-transfected cells. When cultured in such kind of selective medium, cells that were not transfected or were transiently transfected eventually die, and only the stably transfected cells that express the antibiotic resistance gene (for instance, neo) at sufficient levels survive in long-term cultures, thus allowing for the successfully selection and expansion of the desired cells. Descendants of these stably transfected cells therefore will also express the new gene, resulting in a stably transfected cell line.
Although occasionally stable transfection also occurs via the inheritance of nongenomic DNA, maintaining an episomal plasmid in the host nucleus as an extra-chromosomal element [2], the foreign gene becoming part of the genome is the majority of stable transfection, which is the hallmark of stably transfected cells.
Note
1. Usually a single or a few copies of the exogenous DNA is integrated into the genome of a stably transfected cell. For this reason, the expression level of stably transfected genes tend to be lower than that of transiently transfected one
2. Perform control transfection with a vector containing the selectable marker without the gene of interest. If colonies are only obtained from cells transfected with the control plasmid but not from cells transfected with plasmid containing the gene of interest, indicating that the gene of interest might be toxic.
3. It is also important to perform replicate stable transfections in case the transfection fails or the cultures become contaminated.
Types of Transfected Molecule
DNA plasmids are most commonly transfected into cells, but other macromolecules can also be stably transferred. For example, small interfering RNA (siRNA), and microRNA (miRNA) can also be stably introduced into cells when they are delivered as short hairpin transcripts made from a selectable DNA vector. However, RNA molecules themselves cannot be used for stable transfection. In all cases, the reagent needed for transfection ought to be of high quality and relatively pure. Nucleic acids should be free of contamination including proteins, other contaminating nucleic acids and chemicals.
Other Factors
Besides the type of transfected molecules, their purity and quality, transfection reagent or method, the process of cell selection and expansion, there are also some other factors affecting the results of stable transfection. For example, the optimal quantity of DNA to use in the transfection also vary widely, depending on the type of DNA, transfection reagent, target cell type and number of cells. Moreover, selecting the appropriate cell type is a vital decision for a successful stable transfection. Furthermore, cell conditions including confluency percentage is also crucial for transfection. Additionally, assays used to determine the success of stable transfection need to be carefully planned as well. If multiple assays are performed, make sure the techniques you employ are compatible with all assay chemistries.
Benefits and Applications
As stable transfection can establish a permanent alteration in the cell’s genetic makeup and provide persistent expression of the introduced gene through multiple generations, it is useful for production of recombinant proteins and analysis of downstream or long-term effects of exogenous gene expression. Furthermore, stable transfection is one of the most crucial steps to secure the commercial viability of monoclonal antibodies. Although transient trensfection has been employed as a rapid method for antibody production, stable transfection is the preferred option as it allows for greater process consistency and control of the final product quality. Moreover, stable transfection has extensive application in disease modeling, allowing scientists to replicate specific genetic conditions for in-depth studies. It also plays a critical role in functional study of genomics, facilitating a deeper understanding of gene function and cellular signaling pathways.
Conclusions
Transfection serves as a cornerstone in molecular and cell biology, and transfection techniques are vital across diverse scientific disciplines, from basic research to clinical applications and from pharmaceutical development to agriculture, paving the way for innovations in disease research, genetic engineering, as well as biotechnological advancements.
Unlike transient transfection, in which introduced genetic material only persists in cells for a few days, stable transfection introduces gene of interest into cells long-term, supporting continued gene expression in a cell line or certain primary cells without repeated transfections. Its long-term nature makes stable transfection suitable for conducting extended studies or maintaining continuous production of specific proteins and antibodies for various scientific, therapeutic and industrial purposes. The ability to establish a stable cell line makes stable transfection a valuable experimental and clinical research application.
Stable cell lines are important research tools used for various studies including long-term genetic regulation, pharmaceutical drug discovery, compound screening, sustained expression in gene therapy, large-scale protein production, etc. AcceGen offers high-quality Stable Cell Lines using stable transfection, lentiviral transduction and CRISPR-Cas9 techniques to assist our worldwide customers for their variety of research purposes.
References
[1] A. Fus-Kujawa, P. Prus, K. Bajdak-Rusinek, P. Teper, K. Gawron, A. Kowalczuk, A.L. Sieron, An overview of methods and tools for transfection of eukaryotic cells in vitro, Frontiers in bioengineering and biotechnology 9 (2021) 701031.
[2] M.M. Lufino, P.A. Edser, R. Wade-Martins, Advances in high-capacity extrachromosomal vector technology: episomal maintenance, vector delivery, and transgene expression, Molecular Therapy 16(9) (2008) 1525-1538.

Copyright - Unless otherwise stated all contents of this website are AcceGen™ All Rights Reserved – Full details of the use of materials on this site please refer to AcceGen Editorial Policy – Guest Posts are welcome, by submitting a guest post to AcceGen you are agree to the AcceGen Guest Post Agreement – Any concerns please contact marketing@accegen.com