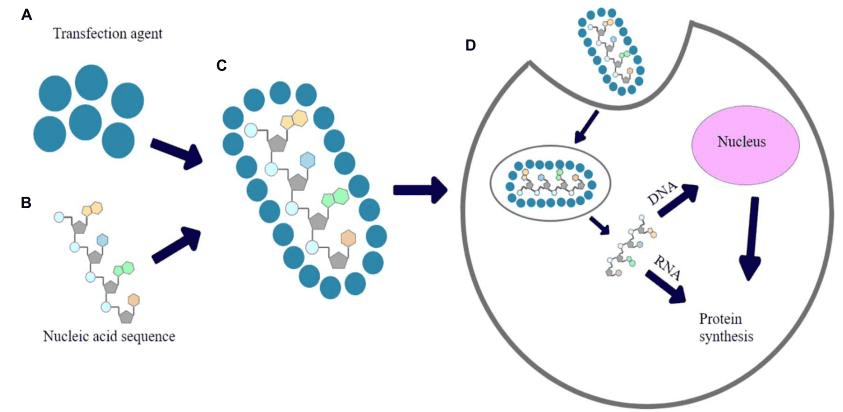
- Human Orbital Fibroblasts
- Human Microglia
- Human Pulmonary Alveolar Epithelial Cells
- Human Colonic Fibroblasts
- Human Type II Alveolar Epithelial Cells
- Human Valvular Interstitial Cells
- Human Thyroid Epithelial Cells
- C57BL/6 Mouse Dermal Fibroblasts
- Human Alveolar Macrophages
- Human Dermal Fibroblasts, Adult
- Human Lung Fibroblasts, Adult
- Human Retinal Muller Cells
- Human Articular Chondrocytes
- Human Retinal Pigment Epithelial Cells
- Human Pancreatic Islets of Langerhans Cells
- Human Kidney Podocyte Cells
- Human Renal Proximal Tubule Cells
Hematopoietic Stem Cells from Bone Marrow
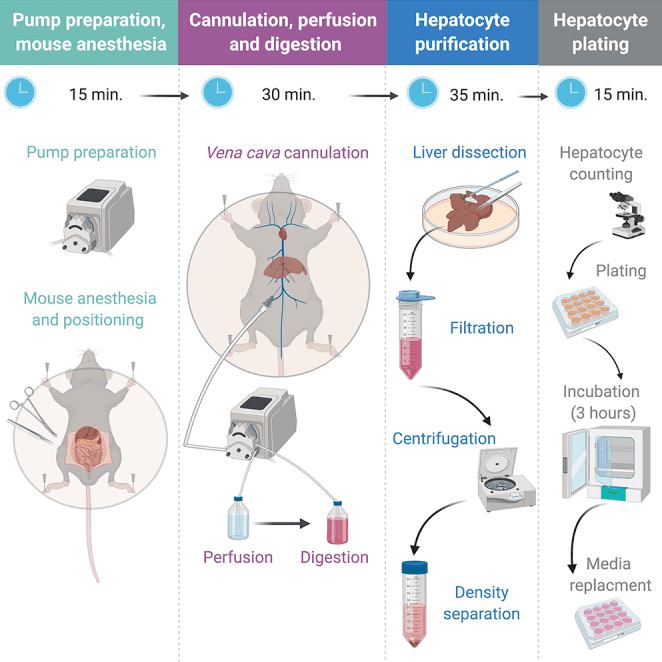
Bone marrow, the spongy gelatinous tissue, is present in the hollow center of bones. It contains hematopoietic stem cells (HSCs), which can produce all types of blood cells which are key components of the immune system. Bone marrow isolation from larger bones, such as the femur and tibia, provides high HSC yield.
Sacrifice of Mice
HSCs can be routinely isolated and purified from bone marrow of mice or other mammals. Take mice for example, this involves sacrificing mice by euthanizing them with a CO2 gas chamber for roughly a minute. After confirming the death of the mouse, end point surgery can be continued. Mice are transferred to an aseptic hood that is not to be used for tissue culture.
Bone Marrow Extraction
To harvest bone marrow, place the euthanized mouse in a supine position and sterilize the skin using ethanol. Douse the mouse in ethanol to sterilize the skin. Remove the loose hair if any. Then cut the abdominal cavity, and remove the skin to expose the hind limbs. After that, break the tibia which is a long bone of the leg, and immediately put it in a 50 mL conical tube containing cold sterile PBS to maintain the physiological conditions. To obtain the thigh bone, dislocate the kneecap and remove the muscles to expose the femur, then cut it off from the bottom of the bone and place in cold PBS as well.

Figure 1. A typical bone marrow harvest location-the mouse hind limb.
Following limb dissection, all skeletal specimens must be maintained at 0-4°C throughout subsequent procedures to preserve cellular viability. Tubes of harvested bones are transferred to a Class II biosafety cabinet pre-treated with UV sterilization and 70% ethanol decontamination.
Decalcified bone processing initiates with mechanical removal of residual soft tissues. Sterile surgical forceps and bone rongeurs are employed to meticulously dissect muscle attachments while preserving bone integrity. The cleaned osseous structures are subsequently immersed in ice-cold isolation buffer (PBS supplemented with 1% fetal bovine serum) to prevent cellular dehydration.
Marrow extraction is performed through mechanical flushing using a 25-gauge hypodermic needle attached to a 10 mL syringe containing chilled isolation buffer. The resultant bone marrow suspension is filtered through a 30 μm nylon mesh to eliminate bone particulates. Throughout this process, the working solution is strictly controlled to be ice-cold using pre-chilled equipment to avoid the viability lose of stem cells.
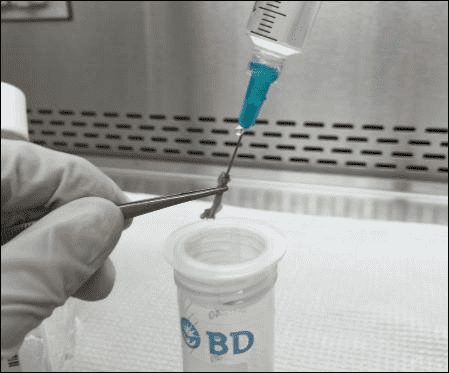
Figure 2. Isolation buffer being pushed through the mouse bone marrow.
Isolation of Cells and Red Blood Cell Lysis
Prior to stem cell enrichment, total nucleated cells from bone marrow need to be quantified via hemocytometer using trypan blue exclusion. Centrifugation at 500×g for 10 min at 4°C facilitates cell pelleting. To remove the red blood cells (RBCs), ammonium-chloride-potassium (ACK) lysis buffer (150 mM NH4Cl, 10 mM KHCO3, 0.1 mM EDTA, pH 7.4) is employed. The lysis of RBCs is very efficient, the treatment only takes 5 min at room temperature. After RBC lysis, the typical number of bone marrow cells obtained from a C57BL/6 mouse femur range from 2.0×10^7 to 5.0×10^7 cells. Usually, a 5% recovery of HSCs from total bone marrow cells is achievable [1, 2].
Purification of Hematopoietic Stem Cells
HSC purification employs the EasySep™ Mouse Hematopoietic Progenitor Cell Isolation Kit (STEMCELL Technologies) following manufacturer specifications. The kit contains Isolation Cocktail, RapidSpheres (magnetic nanoparticles) and Rat Serum. Briefly, the cell suspension is adjusted to 1×10^8 cells/mL in isolation buffer, followed by sequential addition of rat serum (50 μL/mL) and biotinylated antibody cocktail (50 μL/mL). After 15 min incubation at 4°C, magnetic nanoparticles (75 μL/mL) are added. After 10 min incubation at 4°C, add isolation buffer to top up the sample to the desired volume (Top up to 5 mL for samples ≤ 4 mL, top up to 10 mL for samples > 4 mL). Mix by gently pipetting for 2-3 times and incubate for 3 min, allowing unwanted cells labeled with biotinylated antibodies bind to the streptavidin magnetic nanoparticles sufficiently. Subsequently, the labeled cell mixture is ready to be submitted to the magnetic separation using the Big Easy Magnet (for larger volume use) with three washing cycles.

Figure 3. The Big Easy Magnet.
Following the isolation and purification of mouse HSCs, the cells are placed on ice, and then counted for viability and cell number. The final HSC population demonstrates >95% viability by propidium iodide staining. The surface markers expressed on HSCs are characterized by flow cytometry to be Lineage-, c-Kit+ and Sca-1+. The typical isolation efficiency reaches 5.2±0.8% of initial nucleated cells, yielding 2.5×10^6 to 3.0×10^6 functional HSCs per mouse femur as validated by CFU-GM colony formation assays. The purified HSCs can be further differentiated into myeloid and lymphoid lineages.
Conclusion
So far, much of what is known about HSCs comes from the studies performed in mice. Mouse models have helped scientists understand the regulation and function of HSCs. In AcceGen, besides HSC product from mice, we also provide HSCs from other species including rat, monkey and human.
| Cat. No | Product Name | Cell Type | |
|---|---|---|---|
| ABC-SC220G | C57BL/6 Mouse Hematopoietic Stem Cells | Mouse Hematopoietic Stem Cells | +inquiry |
| ABC-SC005G | HighQC™ Rat Embryonic Hematopoietic Stem Cells | Rat Hematopoietic Stem Cells | +inquiry |
| ABC-SC0098T | HighQC™ Non-Human Primate CD34+ Hematopoeitic Stem Cells | Monkey Hematopoietic Stem Cells | +inquiry |
| ABC-SC0085T | HighQC™ Human Cord Blood-CD34+ Hematopoietic Stem Cells | Human Hematopoietic Stem Cells | +inquiry |
| ABC-SC0080T | HighQC™ Human CD34+ Hematopoietic Stem Cells (from bone marrow or liver) | Human Hematopoietic Stem Cells | +inquiry |
| ABC-SC0106 | HighQC™ Human Embryonic Hematopoietic Stem Cell | Human Hematopoietic Stem Cells | +inquiry |
References
[1] D.E. Maridas, E. Rendina-Ruedy, P.T. Le, C.J. Rosen, Isolation, Culture, and Differentiation of Bone Marrow Stromal Cells and Osteoclast Progenitors from Mice, Journal of visualized experiments : JoVE (131) (2018).
[2] P. Yadav, R. Vats, A. Bano, R. Bhardwaj, Hematopoietic Stem Cells Culture, Expansion and Differentiation: An Insight into Variable and Available Media, International journal of stem cells 13(3) (2020) 326-334.

Copyright - Unless otherwise stated all contents of this website are AcceGen™ All Rights Reserved – Full details of the use of materials on this site please refer to AcceGen Editorial Policy – Guest Posts are welcome, by submitting a guest post to AcceGen you are agree to the AcceGen Guest Post Agreement – Any concerns please contact [email protected]